(Image from Institute for Safe Medication Practices)
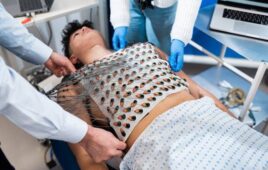
The FDA has relaxed regulations on 21 types of remote patient monitoring devices for the duration of the COVID-19 pandemic to protect healthcare workers from exposure to the virus.
The new guidance might make care for COVID-19 patients safer, as hospitals move patient monitoring equipment into hallways to reduce the need for providers to enter patient rooms.
In a new guidance released on Friday, the agency said it will not object to limited modifications to the indications, claims, functionality, or hardware or software the devices, listed here, as long as they do not impose undue risk.
Companies that make the devices will not have to apply for permission to make the changes covered by the guidance, but the agency wants them to make labeling that clearly describes the use of the device, including lay terminology for those that will be used in the home. That includes making a clear distinction among indications that have and not been FDA-cleared and a general statement about changes that have not been cleared by FDA.
Examples of the modifications are:
- Including monitoring statements related to patients with COVID-19 or co-existing
conditions such as hypertension or heart failure. - Marketing certain devices previously sold only for use in hospitals or other health care
facilities to be used at home. - Altering hardware or software to boost remote monitoring capability.
Changes to hardware or software, such as the addition of wireless and/or Bluetooth capability. should be designed, evaluated and validated according to FDA standards, the agency said. Manufacturers must also include cybersecurity measures.
The full guidance can be found here.