
Douglas Stern, FDA’s Director, Office of Enforcement and Import Operations, Office of Regulatory Affairs
Douglas Stearn, FDA
When FDA identifies that a product it regulates violates the law, it protects the public by working with the manufacturer and distributors to facilitate the product’s recall (i.e., removal from the marketplace or product correction). Among other actions, FDA assures that the public is warned when products present the most significant public hazards, including those recalls associated with an outbreak.
Now, as part of a larger effort to increase transparency, empower consumers, and enhance public health, the FDA is working to alert the public sooner whenever a product has been recalled.
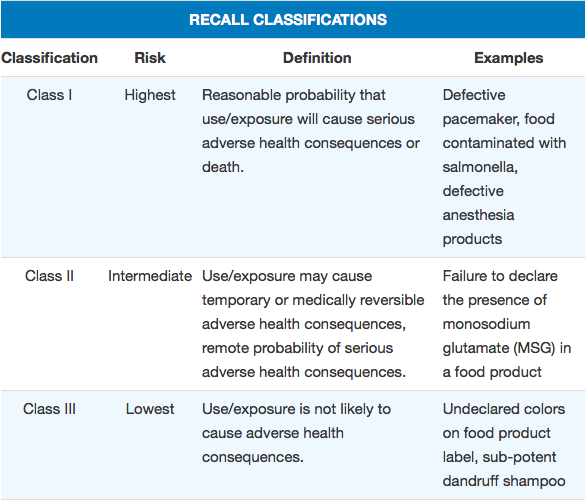
The public’s primary source for recall information is FDA’s weekly, web-based Enforcement Report. Historically, only recalls that have already been classified into one of three categories based on the severity of the hazard have been listed in the report. Through classification, FDA indicates the relative degree of health hazard presented by the recalled product. This enables consumers to better understand the severity of the problem posed by a recalled product so they can take appropriate action. FDA also provides guidance to companies on their recall strategies, taking into account the seriousness of the hazard presented by the recalled product.
However, recall classifications can sometimes take weeks – or even months when FDA needs to conduct a complex evaluation. Such analysis can involve determining whether any diseases or injuries have already occurred, the likelihood that a hazard might occur, or whether vulnerable segments of the population, such as children, are more at risk.
FDA has decided that the public would benefit by having recall information about FDA-regulated products as soon as possible, even though further evaluation remains to be done. Moving forward, FDA will include “not-yet-classified” recalls of human drugs, foods, and veterinary products in the weekly Enforcement Report, even while classification work is still ongoing.
As an example of how this will work, FDA recently posted a “not-yet-classified” recall of a certain lot of animal feed that contained monensin, which can be toxic to cattle at certain levels and could result in cardiovascular illness or death to cattle.
Posting “not-yet-classified” recalls will not affect current FDA protocols for working with companies to ensure that they quickly alert entities in the supply chain as soon as they have identified a problem with their marketed product. Also, FDA will continue to monitor the recalling company’s actions to correct or remove products held by retailers, pharmacies, grocery stores, and hospitals.
In addition to adding “not-yet-classified” recalls of human drugs, food, and veterinary products to the Enforcement Report, FDA recently began posting early summaries of correction or removal actions” involving serious problems with medical devices in the Medical Device Recalls Database. FDA has also enhanced the website’s search capabilities. Returning to the site after viewing “not-yet-classified” recalls is recommended because more information is often made available once a recall has been classified.
The public should recognize that recalls are almost always voluntary. Sometimes a company discovers a problem and recalls a product, while at other times a company acts after the FDA raises concerns. Whether FDA or the company discovers the problem, in every case FDA oversees a company’s recall strategy and assesses the adequacy of the recall.
If a product is believed to pose an immediate, serious hazard, FDA will also act quickly, even before classification is complete, to widely publicize a recall. FDA’s publicity efforts may include press releases, advisories, distribution of alerts to subscribers, and updates to the FDA website. This outreach has proven effective in bringing exhaustive TV, radio, and newspaper coverage about recalls. Increased coverage has occurred in recent years, for example, with certain brands and batches of medicines, injection pens, and contaminated foods such as smoked fish, peanut butter, spinach, frozen vegetables, and ice cream.
The FDA Consumer Update provides more details about the process. Subscribe to the Enforcement Report mailing list at Enforcement Report email subscription. To comment, e-mail enforcementreports@fda.hhs.gov.
Douglas Stearn is the director of the Office of Enforcement and Import Operations at FDA’s Office of Regulatory Affairs.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of MedicalDesignandOutsourcing.com or its employees.




