The U.S. Food and Drug Administration (FDA) in less than a 15-month period, has granted a new and updated 510(k) clearance for two patented technologies made by NICO Corporation that are used in a new approach for brain surgery.
Most recently, NICO received a new clearance for specific neurosurgical indications on its patented Myriad automated tissue removal technology. The new clearance provides specific disease state approval for primary and secondary brain tumors, vascular abnormalities and malformations, and intraventricular tumors and cysts.
NICO also received an updated clearance in July 2015 for its patented BrainPath access technology, now cleared for use in specific disease states such as high grade gliomas and Glioblastoma Multiforme that has only a 15-month survival rate, secondary metastatic tumors, and vascular abnormalities like intracerebral hemorrhage – the deadliest form of stroke. Both the Myriad and BrainPath are used in the rapidly growing brain tumor and hemorrhagic stroke markets that include almost 500,000 combined incidences in the U.S. annually and 5.2 million worldwide.
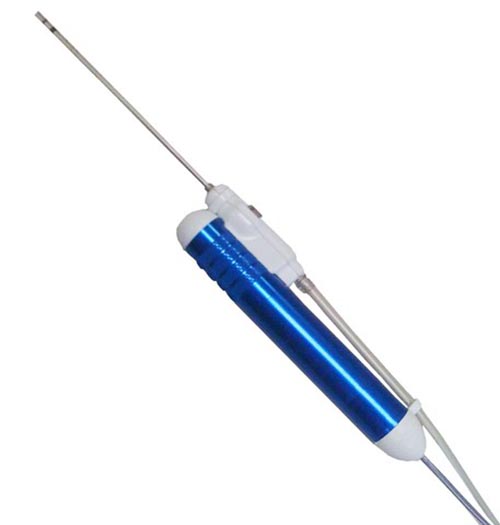
NICO Myriad receives new FDA 510(k) clearance to include use for specific disease states in brain surgery. (Credit: PR Newswire)
The addition of these combined indications by the FDA are key milestones for the company, whose integrated technologies are used as a system in a new approach to brain surgery.
The ability to combine these technologies to gain non-invasive access, perform automated tumor removal and clot evacuation near critical structures, while collecting and preserving removed tissue and clots are helping redefine the notion of “inoperable” brain surgery.
“NICO has spent the last five years bringing together key technology elements to enable minimally invasive neurosurgery for subcortical tumors and clots,” says Jim Pearson, president and CEO of NICO Corporation.
“The goal of NICO has always been to create technology that could create less deficits and faster recoveries in brain surgery. The clinical evidence shows this is possible, and we believe that is directly related to finding less disruptive ways to access the brain that were not possible before. And we believe it will revolutionize the intracranial neurosurgical market.”
Myriad is an automated, multi-functional tool used for precise resection, suction, clot evacuation and tumor removal in neurosurgery, spinal surgery, ENT and otolaryngology.
Nearly 12,000 procedures have been performed using the technology.
“The Myriad system more efficiently preserves the tumor viability allowing me to culture the cells and develop personalized therapies for my patients,” says Lawrence Dickinson, MD, neurosurgeon at Pacific Brain and Spine in Castro Valley, Calif. “This was not possible with older ultrasonic aspirating technologies.”
Dr. Dickinson adds that BrainPath has “revolutionized” his approach to surgical treatment of glial tumors of the cerebrum. “The system allows me to remove these tumors through smaller exposures and with minimal manipulation of the normal brain anatomy,” he says.
Minimally invasive technologies like Myriad and BrainPath are key drivers to more surgical procedures being performed, with documented benefits to patients resulting in fewer days in the hospital, improved recoveries and less cost. Pearson says there is an increasing demand for this same kind of approach involving brain surgery. But until now, the right tools and technologies were not available to offer surgeons an integrated, standardized approach that was better for the healthcare institution, better for the patient, and better economically, he adds.




