The U.S. Food and Drug Administration today allowed marketing of a new tissue expander system for soft tissue expansion in two-stage breast reconstruction following mastectomy and in the treatment of underdeveloped breasts and soft tissue deformities. A patient uses a dose controller to independently inflate the expander.
A tissue expander is a balloon-like device that has a soft, expandable polymer shell and is gradually filled with saline or air. Tissue expanders are typically used prior to breast reconstruction to cause breast tissue and muscle to stretch over time, which creates a space (called a “pocket”) for the breast implant.
The AeroForm device is a wireless tissue expander for patients who choose to have reconstructive surgery following a mastectomy. Most women who have mastectomies to treat or prevent breast cancer are eligible for breast reconstruction.
(Credit: AirXpanders)
“This tissue expander may result in fewer office visits for patients by allowing a patient to partially control their breast tissue expansion,” said Binita Ashar, M.D., director of the Division of Surgical Devices at the FDA’s Center for Devices and Radiological Health. “Patients need to speak with their surgeons about what type of tissue expander is appropriate for them and the benefits and risks of using an expander following their mastectomy.”
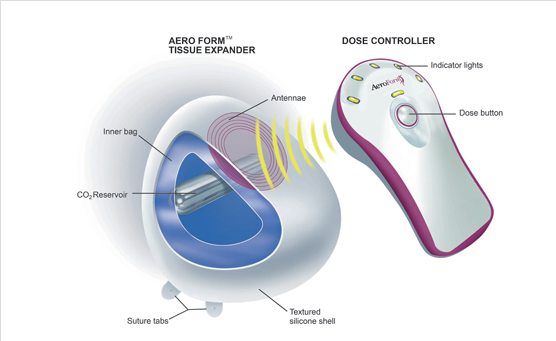
The AeroForm tissue expander system has two main components: a sterile implant with an outer shell made of silicone (called the “expander”) and a remote dosage controller (called the “controller”). The expander contains a reservoir of compressed carbon dioxide. The controller is a hand-held device that communicates with the receiving antenna and electronics located in the expander. The controller is used to communicate to a valve in the reservoir to release carbon dioxide and gradually inflate the expander. The controller is pre-programed to limit releasing a small amount of carbon dioxide once every three hours, up to a maximum of three times per day.
The AeroForm tissue expander differs from available saline-filled tissue expanders. Saline expanders are expanded by the surgeon and use a needle to pierce the skin and inject saline into the expander through a port or injection area. The AeroForm tissue expander is filled with air; there is no need for a needle and the patient has some control over slowly expanding the device at home.
The FDA reviewed results from a clinical trial of 99 patients using the AeroForm expander and 52 patients using the saline expander. The results showed that 96.1 percent of patients using AeroForm expanders and 98.8 percent of patients using saline expanders were able to have their breast tissue successfully expanded and exchanged to a breast implant.
A surgeon must determine whether the patient is a suitable candidate for treatment with the device. Patients must not have any residual tumor at the expansion site and must not undergo magnetic resonance imaging (MRI) while the device is in place. Patients with another electronic implant (e.g. pacemaker, defibrillator, or neurostimulator device) are not eligible for treatment with the AeroForm tissue expander.
The most common adverse events seen in the study were necrosis, seroma, post-operative wound infection and procedural pain. Patients using the AeroForm device in the clinical trials did not report any serious adverse events.
The FDA reviewed the data for the AeroForm system through the de novo premarket review pathway, a regulatory pathway for some low- to moderate-risk devices that are novel and for which there is no legally marketed predicate device to which to claim substantial equivalence.
AeroForm is manufactured by AirXpanders of Palo Alto, California.




