On Feb. 25, 2016, the U.S. Food and Drug Administration released a safety alert for neurosurgery, otolaryngology and orthopaedic specialties. It wrote:
A neurosurgical head holder (skull clamp) system is a device used to secure the patient’s head position during surgical procedures. The system may include a head holder frame that attaches to the operating table, skull clamp, neurosurgical head hold stabilization components, skull pins and other accessories. Based on current information, the FDA believes the overall benefits of these devices continue to outweigh the risks. However, health care providers should be aware of patient-specific characteristics, techniques for safe use, and maintenance of neurosurgical head holder systems to prevent skull clamps from slipping before and/or during surgical procedures.
Summary of Problem and Scope:
From January 2009 to January 2016, the FDA received more than 1,000 medical device reports (MDRs) associated with the slippage or movement of a skull clamp before and/or during surgical procedures, resulting in more than 700 injuries. These reports describe unintended patient movement during surgical procedures that resulted in injuries including skull fractures, facial injuries (bruises and cuts), deep cuts (lacerations), and blood clots (hematoma). Additionally, unintended patient movement has compromised procedures dependent upon head immobilization causing inaccurate stereotaxic navigation and delayed, prolonged, or halted surgical procedures.
The FDA’s review of medical literature and analysis of currently available data suggests that device slippage is not specific to any manufacturer or brand of devices. The causes of skull clamps slipping before and/or during surgical procedures are multifactorial, and may include device performance (e.g., mechanical failure of the skull clamp), device application (e.g., issues with placement of the skull clamp and/or accessories), patient specific characteristics (e.g., thickness and bone quality of the patient’s skull) and lack of maintenance. Some of these risks can be mitigated through device placement considerations, proper use and proper device maintenance.
(Courtesy of the U.S. Food and Drug Administration)
Recommendations:
For Hospital and Health Care Facilities Staff responsible for device maintenance:
- Follow the manufacturer’s recommendations for cleaning, maintenance, and replacement schedule based on device life expectancy.
- Inspect the neurosurgical head holder system and all accessories before and after each use. If any part of the system appears damaged (e.g., breaks, cracks, chips, or other signs of wear) or does not appear to function as expected, remove any defective component or accessory from inventory, and return it to the manufacturer for maintenance and inspection.
For Health Care Providers:
- Follow the manufacturer’s instructions for use and select the appropriate accessories for the neurosurgical head holder system.
- Inspect the neurosurgical head holder system and all accessories before and after each use. If any parts of the system or its accessories appear damaged (e.g., breaks, cracks, chips, or other signs of wear) or do not appear to function as expected, remove the defective component or accessory from inventory and return it to the manufacturer for maintenance and inspection.
- Prior to each use and before fixation, consider applying a force that is comparable to what is anticipated to be used during the surgical procedure to the neurosurgical head holder system. If the system appears unstable, consider obtaining another device.
- Be aware of the correct technique for positioning a patient when using a neurosurgical head holder system including:
- Support the weight of the patient’s head and neck while applying and removing the device to prevent unintended slippage.
- When possible, avoid positioning the skull pins over uneven or fragile bone such as the frontal sinuses, areas of diseased or abnormally thin bone or in areas of varying bone consistencies and thickness, near the orbit, the temporal fossa (squamous temporal bone and thin pterional area), venous sinuses, prior craniotomy bone flap sites, and areas where there are major scalp vessels and nerves to help mitigate slippage.
- Use caution when placing pins in pediatric patients by taking into account different pin designs and possible torque requirements.
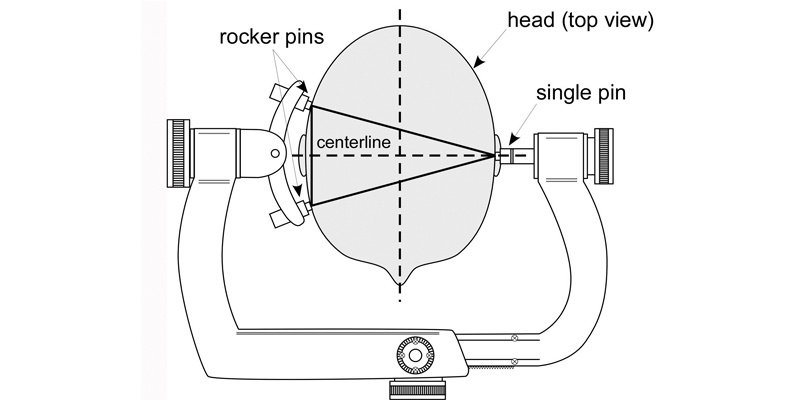
- When possible, utilize the following fixation techniques as shown in Figure 1, which is a graphic illustration of information commonly found in manufacturers’ instructions for use.
- Place the single skull pin as close as possible to the centerline of the patient’s head.
- Place the two rocker pins at equal distance on the opposite side of the patient’s head from the single skull pin.
- Place the angle of the pins (single pin and rocker pins) as close as possible to 90 degrees (perpendicular) to the patient’s skull.
- Apply force appropriate to the thickness and bone quality of the patient’s skull when tightening the neurosurgical head holder system and accessories. Refer to the device manufacturer’s instructions for use for further detail.
- Report any adverse events associated with the use of neurosurgical head holders to the FDA and the manufacturer.
FDA Actions:
The FDA has collected and analyzed data from MDRs, recalls, labeling, and medical literature. Based on current information, the FDA believes the overall benefits of these devices continue to outweigh the risks. The FDA has not concluded that any specific manufacturer or brand of these devices is associated with higher risks than others. The agency will continue to monitor this issue and keep the public informed if significant new information becomes available.
The FDA has proposed a new work item for a voluntary consensus standard to further promote the safe use of these devices across different manufacturers and models. ASTM International Committee F04.25 on Spinal Devices approved the proposal. Publication is anticipated in 2017.




