W. L. Gore & Associates, Inc. announced the first implant of the Gore Tag Thoracic Branch Endoprosthesis (TBE) in the pivotal study. The patient was enrolled by Himanshu Patel, M.D., section head of adult cardiac surgery at the University of Michigan Frankel Cardiovascular Center and co-national principal investigator.
The objective of the study is to determine the safety and efficacy of the TBE in treating lesions of the aortic arch and descending thoracic aorta which includes dissection, trauma, or aneurysm. This pivotal study follows both an early feasibility study conducted in Zones 0-1 with the branch device in the brachiocephalic (BCA) or left common carotid (LCC) arteries and a feasibility study in Zone 2 with the branch device in the left subclavian artery (LSA). The pivotal study will include up to 40 sites across the U.S. that are expected to recruit up to 435 patients, treating all etiologies requiring proximal graft placement in Zones 0-2.
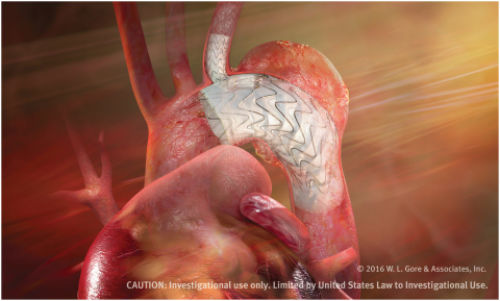
Gore Tag Thoracic Branch Endoprosthesis (Credit: W.L. Gore & Associates, Inc.)
“As a commercially available device, the TBE would offer new less-invasive treatment options for patients with aortic arch disease, which has traditionally challenged both open surgical and endovascular management,” Dr. Patel said. “The conformability of the Gore Tag device combined with the unique side branch design provides a less-invasive treatment of the aortic arch with opportunities for perfusion of the brachiocephalic, left common carotid, or left subclavian arteries.”
Aortic arch disease has traditionally been difficult to treat. The options currently available for the treatment of these pathologies require complex procedures such as open surgery, a hybrid approach with surgical re-vascularization, or combination procedures that use devices in an off-label capacity. The limited options for these patients is one of the reasons that the TBE is the first Gore device to receive the new FDA Expedited Access Pathway (EAP) designation and among the first medical devices to receive this designation in the U.S. This pathway is limited to certain medical devices that demonstrate the potential to address unmet medical needs for life-threatening diseases and offer a meaningful patient benefit compared to existing alternatives.
“Based on the success in the feasibility and early feasibility studies, we are excited about this significant next step to treat a broader range of patients with this innovative device,” said Michael Dake, M.D., Stanford and co-national principal investigator.
Designed for long-term durability, the Gore Tag Thoracic Branch Endoprosthesis is the first off-the-shelf aortic branch device to participate in a pivotal study. With both an aortic and a side branch component engineered to provide perfusion to one arch vessel, the pre-cannulated device was designed to minimize the risk of branch vessel coverage and improve ease of implantation. The branch component also features Gore’s CBAS Heparin Surface, the proven, lasting heparin bonding technology designed to resist thrombus formation.
“The first implant of the TBE in the pivotal study is the latest step in our continuing efforts to offer the broadest endovascular treatment capabilities on the market,” said Ryan Takeuchi, aortic business leader at Gore. “Following a legacy of “firsts” for this device family, we are now the first to advance this therapy into the pivotal phase, allowing the treatment of challenging aortic arch pathologies with an off-the-shelf endovascular device.”
The TBE is part of the growing family of endovascular products that share a mission to effectively treat aortic disease, backed by Gore’s highly rated clinical support team and educational offerings. The comprehensive portfolio of products includes the first FDA approved off-the-shelf iliac branch device, the Gore Excluder Iliac Branch Endoprosthesis (IBE), indicated for the endovascular treatment of common iliac artery aneurysms or aortoiliac aneurysms. To round out the branched portfolio, studies are ongoing in the U.S. and Brazil for the Gore Excluder Thoracoabdominal Branch device.
Products listed may not be available in all markets.
(Source: Business Wire)




