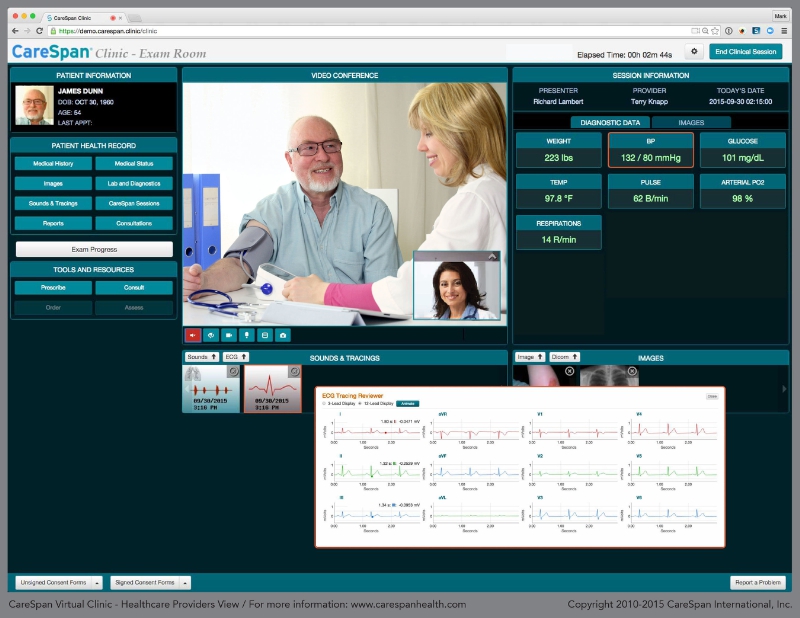
CareSpan and LNI have completed integration between their respective systems and are completing a test suite validating a broad range of Continua devices. (Credit: PRNewsFoto/CareSpan USA Inc.)
CareSpan USA, Inc. (CareSpan) and Lamprey Networks, Inc. (LNI) announced today at the 2015 mHealth Summit that CareSpan will deploy the LNI Health@home hub and Health@home Exchange to move data from hundreds of Continua-compliant devices into the CareSpan Virtual Clinic interface. CareSpan is the first HIPAA compliant digital healthcare platform to incorporate high resolution video communications with a full array of vital signs, breathing/heart sounds, ECG rhythms, medical images and patient records in a workspace designed by physicians for effective and reimbursable care delivery.
LNI will supply its Health@home routers as part of a CarePoint device kit that CareSpan installs at its service locations that include remote work sites such as offshore oil platforms or at Native American community health centers. CarePoints are also being stationed in assisted living centers and corporate offices. Patients at these CarePoints will have standard vital signs, weight/BMI, breathing sounds and images taken with the assistance of an EMT or Physician’s Assistant. Patient data collected during each exam is uniquely identified and securely routed over LNI’s Health@home Exchange to the CareSpan Virtual Clinic for a provider to view and annotate real-time during an exam. Patient data typically transfers from capture to display in five seconds.
“We looked closely at every option in the market that would enable CareSpan to employ one standard API to communicate with a large number of clinical and personal health devices,” said Mark Winter, Chief Executive Officer of CareSpan. “In this highly fragmented medical device industry LNI and the Continua Guidelines stand out as the framework we could rely on to grow our primary care services and specialist consultations at a patient’s workplace, at home or any location where complete healthcare is needed.”
“LNI is delighted that CareSpan chose our solution for industry-standard data exchange and believe that the CareSpan Virtual Clinic will be an ideal demonstration platform to highlight how our hardware and software resources are transforming digital health,” saidMike Mazzola, Chief Executive Officer of LNI. “The CareSpan user interface is the first we’ve seen that unifies all the data a care provider needs to truly meet the standards of an in-person exam. We think that is a key requirement for our industry to grow.”
CareSpan and LNI have completed integration between their respective systems and are completing a test suite validating a broad range of Continua devices. Commercial deployments are anticipated to begin in 2016.




