Medtronic plc and the University of Texas Health Science Center at Houston (UTHealth) announced the first patient enrolled in a clinical study assessing Medtronic’s Valiant Evo thoracic stent graft system for the minimally invasive repair of descending thoracic aortic aneurysms.The international, multicenter, prospective study will include 100 patients in the U.S. and Europe. The news was reported at the 2016 Vascular Annual Meeting of the Society for Vascular Surgery in National Harbor, MD.
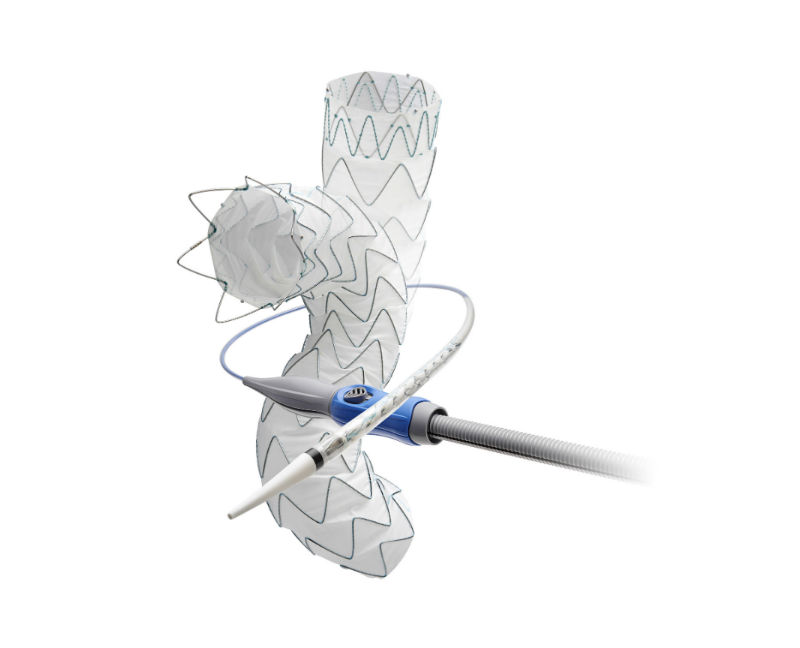
The Valiant Evo thoracic stent graft system. (Credit: Medtronic plc)
Ali Azizzadeh, M.D., FACS, chief of division of vascular and endovascular surgery at McGovern Medical School at UTHealth, is the U.S. principal investigator for the trial and performed the first procedure on a patient with a descending thoracic aortic aneurysm. Azizzadeh reported the first patient treated in the investigational device exemption trial is an 88-year-old male who is doing well post-treatment.
The Valiant Evo system is a lower profile evolution of the Valiant Captivia system, which has treated more than 85,000 patients globally. Valiant Evo is designed to deliver a minimally invasive treatment option to a greater number of patients with an 18F and 20F outer diameter profile for most size configurations, proximal closed web and FreeFlo configurations with tip capture, and high stent graft conformability. Additional features include broader tapered offerings and longer lengths for anatomical customization, in addition to an improved ergonomic delivery system.
In the United States, the Valiant Evo is an investigational device and not yet approved for commercial use.
Approximately six out of 100,000 people globally experience a thoracic aortic aneurysm, a blood-filled bulge or ballooning of the aorta that runs through the chest and can lead to a life-threatening rupture and hemorrhage if not treated. Most people do not have any symptoms. However, risk factors include smoking, obesity, heredity, injury, or other disease.




