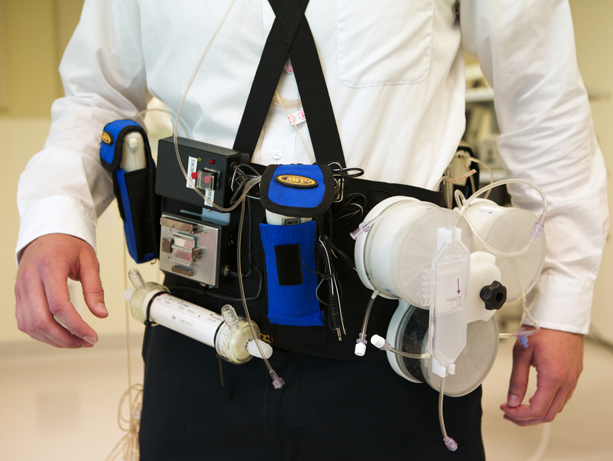
The WAK is a belt-like device that miniaturizes the functions of a 300-pound dialysis machine down to 11 pounds, and uses just a half liter of water as opposed to 40 gallons per session.
The Food and Drug Administration (FDA) has awarded its new Expedited Access Pathway (EAP) to the first Wearable Artificial Kidney (WAK) invented by UCLA/Cedars Sinai Nephrologist Dr. Victor Gura of Beverly Hills, CA. The EAP designation signals FDA support for expediting the availability of this device to the 600,000 patients suffering from End-Stage Renal Disease (ESRD) in the United States alone. This development also offers new hope for the millions of hemodialysis patients around the globe.
The first U.S. human trials of the externally worn WAK have been conducted at the University of Washington at Seattle — the details of which will be presented at the American Society of Nephrology’s annual conference ASN Kidney Week in San Diego, CA on 11/7/15. Successful human trials have also been conducted in the U.K. and Italy. The WAK represents the first material advancement in dialysis technology in several decades. The mortality rate for ESRD patients in the U.S. is 20% and rising.
“We are thrilled that the FDA has awarded the Wearable Artificial Kidney with its new Expedited Access Pathway,” said Dr. Gura. “The WAK has been in development for 14 years, and this designation brings us one vital step closer to making it available to the public. Soon, we hope to be improving the lives of hemodialysis patients in the U.S. and around the world with the WAK.”
Traditional dialysis not only makes a normal life difficult for patients because they need to be strapped to a kidney dialysis machine for 9-12 hours per week but because it severely restricts their diets. With the WAK, patients can expect to enjoy many of the foods that have been forbidden to them for years; improve their quality of life; reduce hospitalizations and increase longevity.
What Is the WAK Device?
The WAK is a belt-like device that miniaturizes the functions of a 300-pound dialysis machine down to 11 pounds, and uses just a half liter of water as opposed to 40 gallons per session. It is battery-operated, user-friendly and lightweight allowing for freedom of movement for patients. Much like a normal kidney, the WAK removes excess fluids and toxins from the blood at a natural rate, and provides patients with continuous treatment (as opposed to intermittent) allowing them to achieve a quality of life closer to that of a healthy person.
FDA Notification RE: The EAP Program and the WAK
According to the official notification from the FDA’s Center for Devices and Radiological Health (CDRH) dated Oct. 7, 2015 entitled, “Expedited Access for Premarket Approval and De Novo Medical Devices Intended for Unmet Medical Need for Life Threatening or Irreversibly Debilitating Diseases or Conditions”: “We are pleased to inform you that your (WAK) device and future premarket submission meets the criteria and has been granted EAP designation and priority review processing. The proposed indication includes use in “’kidney failure patients that meet standard criteria for renal replacement therapy by hemodialysis.”




