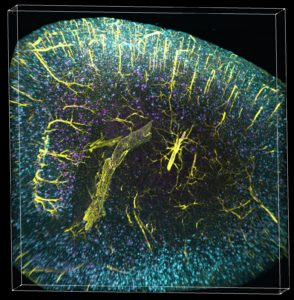
The scientists are now also able to co-visualize in 3D simultaneously amyloid plaques together with two other parameters (e.g. tau, microglia and vasculature).
The Fisher Center for Alzheimer’s Research Foundation is proud to announce they have funded the conception of state-of-the-art technology created by the Fisher Center scientists.
As published in the scientific journal Cell Reports, under the direction of Dr. Paul Greengard, Dr. Marc Tessier-Lavigne, and spearheaded by Dr. Marc Flajolet, Fisher Center scientists created technology that allows the visualization of amyloid plaques as well as other Alzheimer’s hallmarks, such as tau, vasculature and microglia activation, in a large volume, in an entire mouse brain with the potential application of frozen human brain samples.
“We are proud that the funding we provide has resulted in innovative, never before seen imaging of what causes Alzheimer’s disease,” said Kent L. Karosen, President and CEO Fisher Center for Alzheimer’s Research Foundation. “The Fisher Center scientists are working diligently to better understand the cause and cure of the disease and with the ability to visualize the causes of Alzheimer’s, we’re one step closer to a cure.”
Using the iDISCO visualization method involving targeted molecular labeling, tissue clearing and light-sheet microscopy, the Fisher Center scientists gained unprecedented access to intact Alzheimer’s disease mouse brains and studied in detail amyloid plaque content in five major brain regions, at different ages.
The scientists are now also able to co-visualize in 3D simultaneously amyloid plaques together with two other parameters (e.g. tau, microglia and vasculature). Volume imaging coupled with automated detection and mapping enables precise and fast quantification of plaques within the entire intact mouse brain, a much faster and more economical alternative to standard beta amyloid plaque labeling.
Further analysis of archived human brain tissues from patients led to the remarkable discovery of large 3D amyloid patterns that the researchers of the Fisher Center called 3D amyloid patterns (TAPs) and that measure up to 27 cubic millimeters. In these human samples, contrary to the mouse brains, scientists also observed a larger diversity of the amyloid plaques in terms of size and 3D shape.
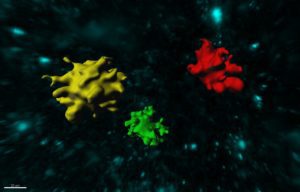
Further analysis of archived human brain tissues from patients led to the remarkable discovery of large 3D amyloid patterns that the researchers of the Fisher Center called 3D amyloid patterns (TAPs) and that measure up to 27 cubic millimeters.
These intriguing differences between animal models and human samples might highlight a novel trait of, and specific to, the development of Alzheimer’s disease in humans. The combination of these parameters provides access to an expansive set of possibilities for pre-clinical studies and for further pathological exploration. Larger studies focusing on patient samples will allow scientists to confirm the existence of TAPs and retrospective studies performed with clinicians might lead to the emergence of a disease classification.
Fisher Center for Alzheimer’s Research Foundation
alzinfo.org




