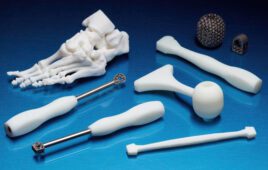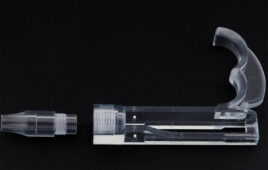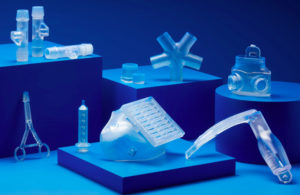
Parts made with Formlabs’ BioMed Clear resin [Image courtesy of Formlabs]
The new material families and resins are meant to provide more options for designers and engineers in fields including:
- Healthcare — with a new line of BioMed biocompatible resins including BioMed Clear resin and BioMed Amber resin;
- Engineering, product design, and manufacturing — with a new family of flexible and elastic resins consisting of Flexible 80A resin, a reformulation Formlabs’ present Flexible resin, and Elastic 50A resin.
- Dental — with a novel material for temporary restorations created through a Materials Partnership Platform with BEGO, as well as a new resin for manufacturing custom impression trays.
Formlabs described its BioMed Clear resin as a strong, hard resin for biocompatible applications requiring long-term skin or mucosal membrane contact. BioMed Clear is USP Class VI certified and suitable for applications that require wear resistance and low water absorption over time. It’s possible to sterilize parts created with the resin according to CDC standards. The material is supported with an FDA Device Master File.
BioMed Amber resin is a rigid material ideal for biocompatible applications requiring short-term skin or mucosal membrane contact, according to Formlabs. Parts printed with BioMed Amber resin are compatible with common solvent disinfection and sterilization methods.