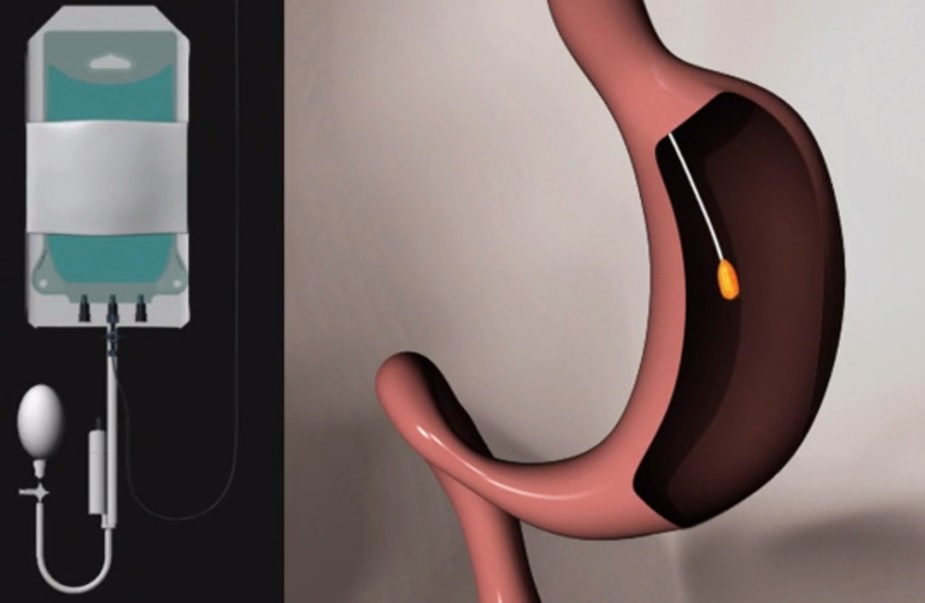
Allurion Technologies’ gastric balloon pill (Credit: Allurion Technologies)
A new gastric balloon that can be swallowed like a pill and then filled while in the stomach, helped patients lose more than 37 percent of their excess weight over four months, according to new research presented here at Obesity Week 2015, the largest international event focused on the basic science, clinical application and prevention and treatment of obesity. The weeklong conference is hosted by the
American Society for Metabolic and Bariatric Surgery (ASMBS) and The Obesity Society (TOS).
Allurion Technologies, the manufacturer of the device called Elipse, which is not yet commercially available, is studying what it says is the “first procedureless gastric balloon” in patients with a body mass index (BMI) of 27 or more.
The treatment involves patients swallowing a capsule that quickly dissolves in the stomach to reveal a deflated gastric balloon inside. With a thin catheter attached to the device, but long enough to remain outside the patient’s mouth, a physician fills the balloon with fluid (550 mL) to about the size of a grapefruit. The catheter is then removed, while the balloon remains in the stomach for four months. At that point, a valve designed to open on its own, allows the balloon to empty and be excreted naturally from the body, eliminating the need for endoscopy or another procedure.
“Like other gastric balloons, the mechanism of action of Elipse is likely multifactorial and includes increased satiety from the reduction of available space in the stomach, delayed gastric emptying, and changes in hormones that control hunger and appetite,” said Ram Chuttani, MD, study co-author and director of Endoscopy and chief, Interventional Gastroenterology at Beth Israel Deaconess Medical Center in Boston. “Our findings demonstrate that Elipse provides individuals and their caregivers with a safe, effective, and non-invasive weight loss intervention that does not require surgery, endoscopy, or anesthesia.”
Researchers presented interim results for the first 34 patients of a multi-center study that showed individuals lost an average of 22 pounds after four months or 37 percent of their excess weight. Patients also saw improvements in triglycerides and hemoglobin A1c (HgbA1c) levels, risk factors for heart disease and diabetes. Similar to other gastric balloons, the most common adverse events reported were nausea and vomiting.
NinhT. Nguyen, MD, immediate past president of the ASMBS and vice-chair, UC Irvine Department of Surgery and chief of gastrointestinal surgery, who was not involved in the study said the device is not a permanent solution to weight loss, but has the potential to help those individuals who are overweight or have obesity and are not candidates for bariatric surgery.
“New treatment options are being studied and approved for the treatment of obesity, which is good news for our patients and the healthcare professionals involved in their treatment,” said Dr. Nguyen, who was not involved in the study. “For many struggling with their weight, procedureless gastric balloon devices may serve as a treatment option that bridges the gap between weight-loss drugs and surgery.”
The U.S. Food and Drug Administration (FDA) this year alone approved the Orbera Intragastric Balloon from Apollo Endosurgery and theReShape Integrated Dual Balloon System from ReShape Medical. Both devices are indicated for adults with BMIs between 30 and 40 who could not lose weight through diet and exercise alone. The FDA also approved three weight-loss drugs since 2012.




