Coronary or peripheral bypasses are the most frequently performed vascular operations. Although one million patients per year around the world undergo this intervention its failure rate reaches 50 percent because of poor vessel healing, leading to vessel graft occlusion.
To improve the outcome of bypasses, researchers from the University of Geneva (UNIGE) worked together with medical doctors from the Lausanne University Hospital (CHUV) to develop GeM, a gel containing microparticles enabling the controlled release of a drug inhibiting cellular over-proliferation.
Administered locally, directly on the bypass graft during surgery, this preventive treatment will reduce the risk of obstruction recurrence. The research was published in The Journal of Controlled Release.The vascular bypass allows a blocked artery to be bypassed by implanting a vessel removed from the patient, and this is done in order to create a deviation in circulation. However, in 50 percnet of cases, excessive vascular cell proliferation, called hyperplasia, occurs around the suturing site of the transplanted vessel.
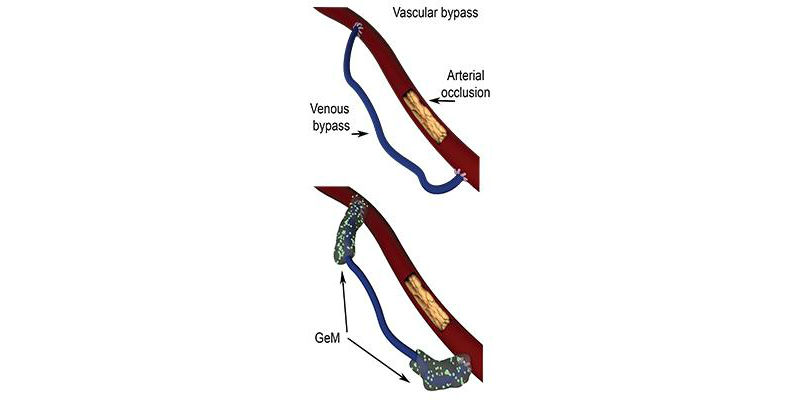
(Credit: University of Geneva)
Hyperplasia then leads to a decrease in blood flow within five years following the operation, requiring a new surgery. Today, doctors prescribe atorvastatin (ATV) orally against hyperplasia. However, this route of administration is not adapted to this pathology.
This led Dr. François Saucy’s team from CHUV to joine forces with Florence Delie and Olivier Jordan, researchers at the Department of Pharmaceutical Sciences at UNIGE Faculty of Science. The goal was to find a way for local administration of ATV that will remain available over a long time period, in order to overcome the disadvantages of high dosage taken orally.
“We immediately thought of a gel mixed with ATV, being directly applied on the vessel during surgery,” Delie said. “Being viscous, it remains in place and enables local release. But, we had an important challenge — doctors recommended the presence of the drug over a period of four weeks to avoid hyperplasia development, while the gel is only effective for three days.”
Researchers from UNIGE consequently added polymer microparticles containing ATV to the original formulation. The microparticles carry the encapsulated drug and gradually dissolve into the gel, releasing ATV over one month.
“It is about a controlled release system: The right dose at the right place, with the correct release profile,” Jordan said. “We discovered that this formulation only works using the combination of, on one side, quickly released ATV mixed into the gel and on the other side, the microparticles, which act over the long term.”
The researchers siad its use on patients is contemplated in five to 10 years.




