GI View Ltd., developer of advanced GI screening systems, announced today that it has received FDA 510(k) clearance for the new Aer-O-Scope Colonoscope System, a disposable, self-propelled, joystick-controlled, easy-to-use colonoscope system, now with therapeutic access. The new system has two working channels that enable therapeutic access using standard tools, such as snares and forceps, to take biopsies or perform polypectomies.
“Today’s news marks the achievement of an important regulatory milestone. The new Aer-O-Scope system with therapeutic access has many significant clinical benefits including enabling physicians to more easily, effectively, and efficiently identify and remove polyps and prevent colon cancer,” says GI View CEO Tal Simchony, Ph.D. EE. “We are now working on U.S. market introduction of the Aer-O-Scope. Post market studies are also in the plans.”
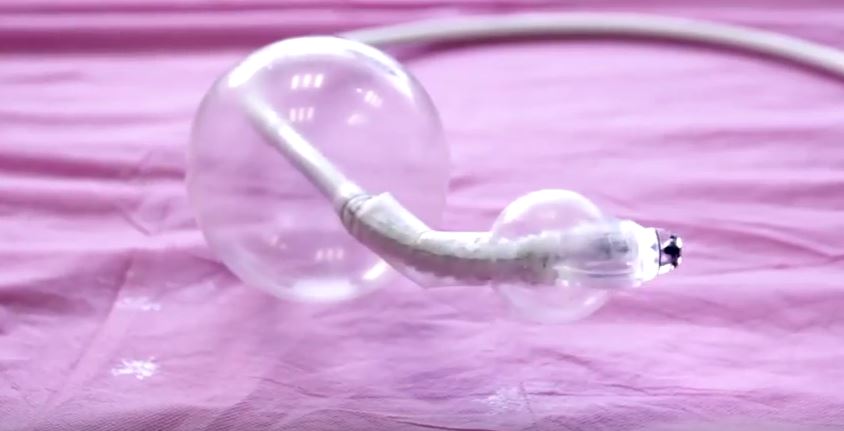
(Image credit: screengrab from Aer-O-Scope promotional video)
The Aer-O-Scope is the first colonoscope to provide a 360° omni-directional visualization of the colon to detect polyps behind folds. There is also no risk of contamination or disease transmission between patients from the device as it is to be used only once and then disposed. The Aer-O-Scope employs a soft multi-lumen tube designed to significantly reduce pressure on the colon wall, which in turn, increases patient safety. The tube is also hydrophilic, which reduces the friction between bowel and scope by more than 90%. Patient safety and comfort as well as physician ease of use are further maximized by the system’s self-propelled intubation, created using balloons and low pressure CO2 gas. As the system is joystick controlled it is also extremely simple to operate and requires minimal training. Like all colonoscopes, the Aer-O-Scope provides insufflation, irrigation, and suction.




