In February 2016, Global Technologies of Spring Lake became the first company in the world to receive MedAccred Cable and Wire Harnesses accreditation.
MedAccred is an innovative new industry managed supply chain oversight program that bolsters patient safety. It does this through clarification of requirements and better identifying how they apply to critical processes used in the production of medical devices.
Global Technologies is now officially the first company to be fully compliant with the MedAccred Audit Criteria for Cable and Wire Harness Assemblies (AC8121), which were developed by the MedAccred Cable and Harness Assemblies Task Group. The members of the Task Group come from companies including Philips, Stryker, GE Healthcare and Baxter Healthcare. Global Technologies was also involved in the development of the audit criteria, which have been developed in accordance with the Class 2 and Class 3 requirements of IPC/WHMA-A-620, J-STD-001 and other industry standards.
Global Technologies was an early adopter of the program and participated in the Task Group from its inception. Jeff Olds, CEO of Global Technologies, said, “We are extremely proud to be the first company to achieve the accreditation from MedAccred for Cable and Wire Harnesses. A MedAccred audit is a rigorous assessment of critical process capability and compliance to customer requirements and industry standards. I am delighted to be able to say to all our customers that Global Technologies’ capabilities and compliance status have been validated through this audit process. We believe MedAccred will become an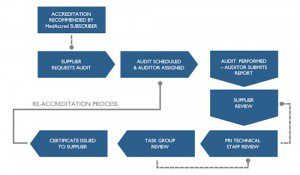 important part of the medical device industry’s approach to supply chain oversight and it is fantastic to be recognized as being at the forefront of this effort.”
important part of the medical device industry’s approach to supply chain oversight and it is fantastic to be recognized as being at the forefront of this effort.”
 important part of the medical device industry’s approach to supply chain oversight and it is fantastic to be recognized as being at the forefront of this effort.”
important part of the medical device industry’s approach to supply chain oversight and it is fantastic to be recognized as being at the forefront of this effort.”
Joe Pinto, Executive Vice President and Chief Operating Officer at the Performance Review Institute said, “We would like to thank Global Technologies for their ongoing and active participation in the MedAccred program. It is because companies like Global Technologies are willing to step forward and be the first to conduct an audit at their facility that MedAccred is able to start demonstrating the value it offers the whole industry. Being the first to achieve accreditation is not easy and everyone at Global Technologies should be very proud of their new status as the first MedAccred Cable and Wire Harnesses accredited supplier.”
Benefits of MedAccred:
- provides consistent/standardized critical process accreditation accepted by leaders in the Medical Device Industry resulting in fewer redundant onsite audits by multiple OEMs
- conducts in-depth critical process audits that are compliant and consistent to accepted industry/technical standards and conducted by Subject Matter Experts
- provides greater visibility of the supply chain to all levels and sub-tiers that provide critical processes, consistent with regulatory requirements (for example, FDA, ISO 13485, MDD, and so on)
- improves flow down of OEM requirements to sub-tier suppliers
MedAccred
p-r-i.org/medaccred
Global Technologies
globaltec.com



