By Ronelle Decker, Senior Business Development Manager – Omniseal Solutions™ Life Science
High Performance Life Science Material Options & Solutions
Omniseal Solutions™ is a leading global engineering company specializing in the design and manufacture of precision sealing and material solutions for critical applications in challenging environments. For over 65 years, they have provided life science solutions that support the diagnosis and treatment of diseases, protect surgical devices, and enable home health mobility.
In an exclusive white paper, the Omniseal Solutions’ life science team explains the advantages of USP Class VI certification as well as the challenges that the industry faces in meeting these standards, i.e., requirements, tests, and processes involved in obtaining this certification. Using high performance materials such as Omniseal Solutions’ Rulon® and other polymers can help solve the challenges in this industry.
The USP Class VI Certification: Tried, True & Tested
Biocompatibility is critical in the life science industry to ensure medical device safety and clinical success.
Achieving USP Class VI accreditation can be highly advantageous for medical manufacturers: reducing waste, increasing profitability, and building trust. Furthermore, USP Class VI certification has a qualified standing worldwide, making it excellent for a wide range of medical components and their end-users.
Our Advanced & Certified Material Solutions
With a global presence and local resources, Omniseal Solutions™ provides precision solutions with tight tolerances, working closely with key life science customers to ensure that the right material fits the right solution.
See Our Certified USP Class VI Material Solutions:
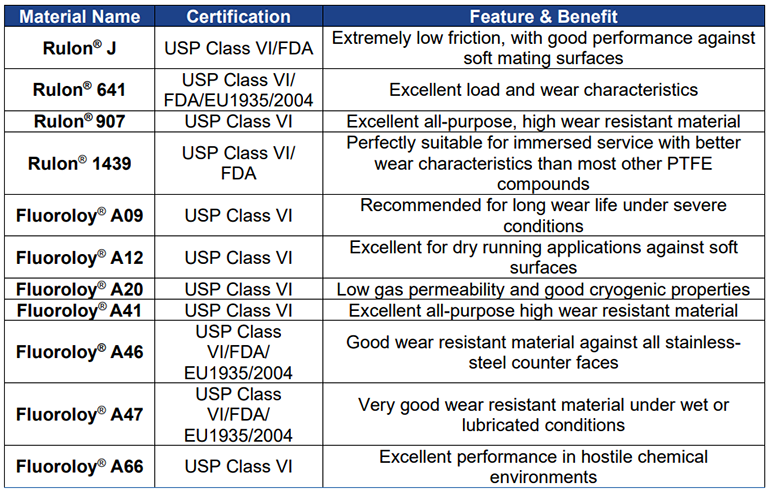
The above table lists 11 Rulon® and Fluoroloy ® USP Class VI certified materials for your engineering needs, which is unique in that most other businesses may only have three or four certified materials in their solutions portfolio. You need knowledgeable subject matter experts (SMEs) and experienced engineers who can find the right balance between cost investment / savings and the necessary protection for medical device manufacturers. All of the above materials are thoroughly tested and validated, ensuring safety, high performance, and trust. They are available globally and produced with unique formulations.
“With USP Class VI certification as a testament to their excellence, our advanced materials offer an unparalleled advantage to our customers in the medical device industry. Their exceptional adherence to strict quality standards ensure that our customers stay at the cutting edge of medical device advancements.” Explains Ronelle Decker, Life Science Senior Business Development Manager at Omniseal Solutions™.
“To provide global convenience and accessibility, Omniseal Solutions™ rigorously tests Class VI materials from multiple plants across the world, ensuring a robust supply chain.”
Our Life Science Applications
Find out how Omniseal Solutions’ precision and performance materials go beyond in pharmaceutical applications to meet extremely demanding performance requirements such as chemical compatibility and biocompatibility.
Pharmaceutical Bioreactor Application Using Omniseal Solutions’ Polymer Materials

Click the link below to download the FREE WHITE PAPER and gain more insights on how the use of USP Class VI certified materials can improve production efficiency, reduce waste, increase profitability, and ensure patient safety.
Click Here to Download the Free White Paper Now
At Omniseal Solutions™, we are dedicated to design engineering our sealing and material solutions for existing and future stringent requirements to protect our customers and the patients they serve.
If you have any questions or would like to discuss a potential project on a challenging application, please do not hesitate to CONTACT OUR EXPERTS.
Sponsored content by Saint-Gobain