Gordian Surgical recently received CE clearance for its TroClose1200, an innovative trocar with integrated closure system for the suturing of abdominal wall incisions during laparoscopic surgical procedures.The company announced the completion of registration and receipt of CE approval to begin marketing the TroClose1200. Together with this certification, Gordian also received ISO13485 certification.
Gordian Surgical’s TroClose1200 acts both as a trocar, through which surgical instruments enter the abdomen, and to close internal incisions made during surgery, delivering “two-in-one” functionality. Currently, surgeons insert sutures in a time-consuming and difficult process at the end of the procedure or they close internal incisions with the use of an additional device. Using the TroClose1200’s uniquely designed release mechanism, sutures are inserted into the tissue at the beginning of the procedure and anchored to remain in place throughout the operation, allowing incisions to be closed easily and quickly upon removal of the TroClose1200.
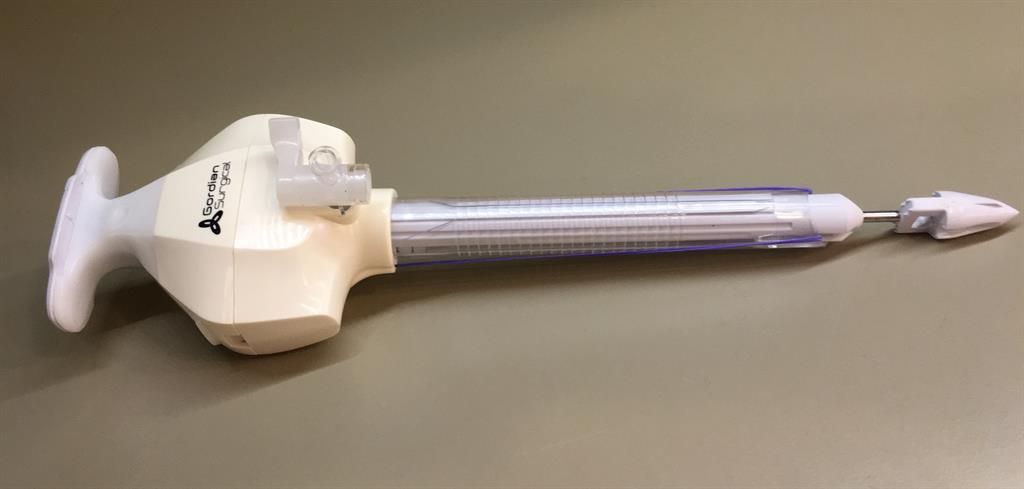
(Image credit: Gordian Surgical)
Gordian has started human trials to demonstrate safety and efficacy and has, to date, performed 34 successful laparoscopic procedures using the TroClose1200, including hysterectomy, cholecystectomy (gallbladder removal), hernia repair, and sleeve gastrectomy. The surgeries were performed by four different surgeons in two medical centers in Israel and abroad. Gordian expects to complete 50 additional procedures as part of the trial by the end of 2016.
Gordian CEO Zvi Pe’er remarks: “Receiving CE Mark certification allows us to move to the next phase and start sales, a critical step in our development. We expect to begin sales during the first half of 2017.”




