Gordian Surgical announced it received FDA regulatory clearance for its TroClose1200, an innovative trocar with integrated closure system for the suturing of abdominal wall incisions during laparoscopic surgical procedures. The FDA approval follows Gordian’s receipt of CE Mark certification as announced in September.
Gordian Surgical developed TroClose1200 to give surgeons “two-in-one” functionality: the device acts both as a trocar, through which surgical instruments enter the abdomen, and a device to close internal incisions made during surgery. The sutures are inserted into the tissue at the beginning of the procedure and anchored to remain in place throughout the operation, allowing incisions to be closed easily and quickly upon removal of the device.
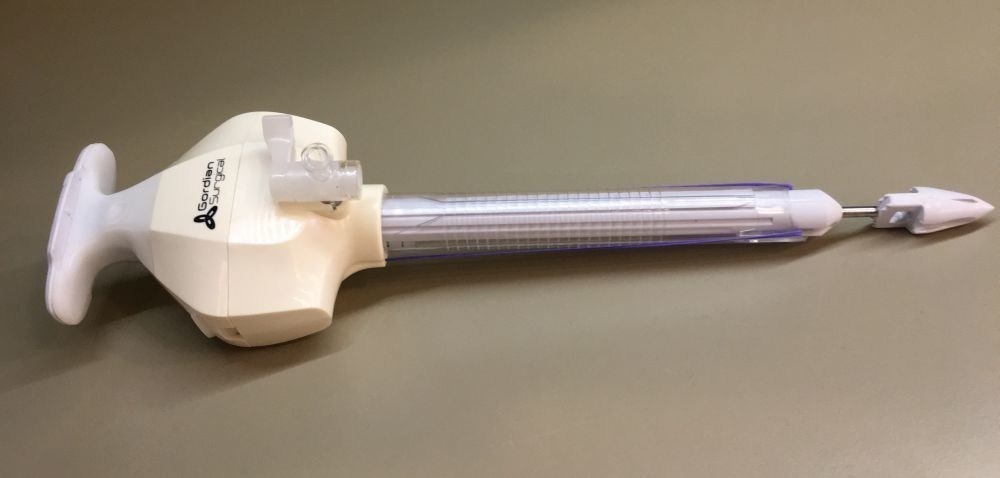
(Image credit: Gordian Surgical)
Gordian began human trials in 2016 to demonstrate safety and efficacy of TroClose1200 and to date has completed all 50 laparoscopic procedures in the clinical phase. Additionally, the Company performed the first seven cases, defined as post-marketing surveillance, in Europe, including at the prestigious IRCAD in Strasbourg, France and during a live surgery that was broadcast to surgeons at the 32nd Annual Conference of the German Association for the Study of Obesity (DAG) e.V. in Frankfurt.




