
William Conley, sales manager of the extrusion division at Guill, demonstrates the single-point concentricity adjustment of the Micro-Medical crosshead. [Image courtesy of Guill Tool & Engineering]
Officials at Guill Tool & Engineering (West Warwick, R.I.) think they could bring medical tubing extruders’ capabilities to new heights through their recently released Micro Medical crosshead.
The Micro Medical crosshead — which Guill touted at MD&M West in February — is an extrusion crosshead with a patented single-point concentricity adjustment. Guill officials described the single-point adjustment as a unique innovation for the extrusion of thin-walled jacketing and precision ID/OD tubing. Unlike previous crosshead designs with four adjustment bolts 90° apart, there is one bolt that controls 360° of adjustment.
The Micro Medical’s design prevents problems with downtime or quality that sometimes occurred with the standard crossheads, which had operators loosening one bolt and tightening an opposing bolt 180° apart, William Conley, sales manager of the extrusion division at Guill, told Medical Design & Outsourcing at the show.
“If the operator decides to take a shortcut and not loosen the opposing bolt, often the bolt you are tightening will break in the die holder retainer. This will require the line to be shut down to repair the damage. Or if the operator does not inform his supervisor, you may run defective product for an extended period of time,” Conley said.
The single-point adjustment, working in conjunction with a two-stage clamping system, solves the problem, Conley said.
“The two-stage clamping system allows you to pre-load the tension required to move the die holder. The single concentricity bolt is rotated to the high spot in the wall, and a simple adjustment with a 4-in. wrench will resolve the issue,” he explained.
“It’s much simpler for the operators,” Conley added. “There’s less downtime because you don’t have as many broken components.”
The single-point adjustment system has an added benefit: Adjustments are no longer restricted to 90° increments, according to Conley.
“You will have access with full 360° of movement,” he said.
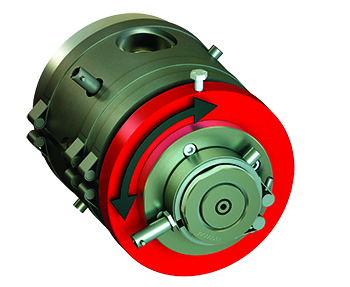
Guill Tool & Engineering officials tout the Micro Medical crosshead’s single-point adjustment as a unique innovation for the extrusion of thin-walled jacketing and precision ID/OD tubing. [Image courtesy of Guill Tool & Engineering]
- The crosshead uses micro-fine adjustment screws for precise concentricity adjustment during extrusion of thin-walled and precision ID/OD medical tubing. Concentricity precision reaches 0.008 in. or finer per revolution, according to Guill.
- The single-point crosshead includes a patented cam-lock deflector for quick changeovers. The deflector has a a residence time of 1 min. at 0.5 lb/hr material flow. It has optimized usage with extruders measuring 0.5 in. and 0.75 in., and a max die ID of 0.25 in.
- The Micro Medical crosshead also offers great user flexibility, according to Guill. It accepts both vacuum and micro-air accessories and is also ideal for pressure and sleeving applications. Fluoropolymer designs are available upon request.
Because there’s now a single point to adjust concentricity, it could become much easier down the road to automate the extrusion process, Conley said.
“Ultimately what we’re looking to do is at some point is get automatic feedback from the engaging system,” he said. “So basically what they’ll do is they’ll be able to extrude the product, it goes into the tank, you’ll see what the finished product is as it comes out of the tank, and it’ll tell you where the error is in concentricity, where the adjustment has to take those that can feed a signal back to an encoder in a servo motor, move that around to actually adjust concentricity per the engaging system.
“It basically takes the operator 100% out of concentricity,” he said.




