An experimental model uses genetics-guided biomechanics and patient-derived stem cells to predict what type of inherited heart defect a child will develop, according to authors of a new study in the journal Cell.
A multi-institutional team developing the technology – and led by the Cincinnati Children’s Heart Institute – reports May 19 it would let doctors intervene earlier to help patients manage their conditions and help inform future pharmacologic treatment options. In laboratory tests, the model accurately predicts whether mouse models and stem-cell derived heart cells from human patients will develop a hypertrophic or dilated cardiomyopathy.
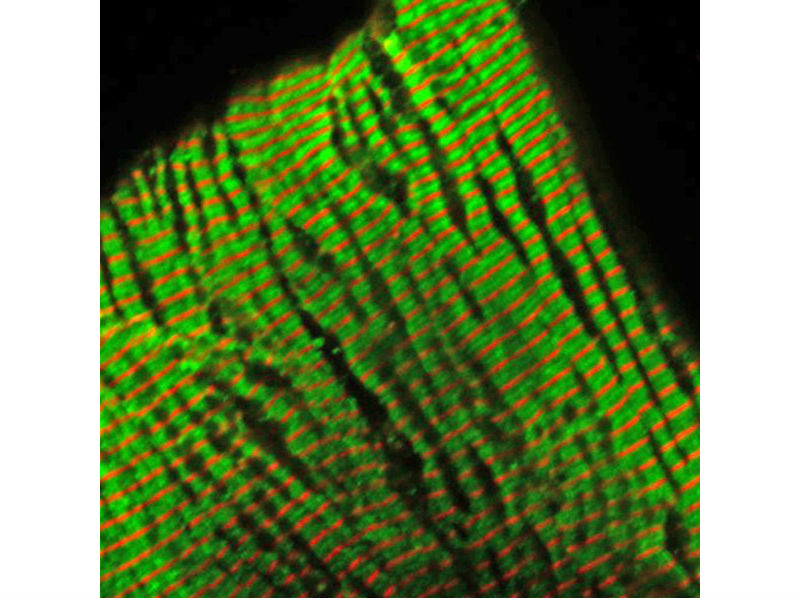
With cells illuminated by fluoresced antibodies, this microscopic photograph of sarcomeres – the part of heart muscle that generates tension and contraction – shows a mutant troponin protein used to study inherited cardiomyopathy. Researchers at Cincinnati Children’s Hospital Medical Center and the Howard Hughes Medical Institute have developed a computational algorithm that allows them to measure biochemical and physical changes and predict if heart tissues will develop a hypertrophic cardiomyopathy or dilated cardiomyopathy. Researchers report May 19 in the journal Cell the technology could one day allow physicians to intervene earlier to help patients with mutations that cause inherited cardiomyopathies. (Credit: Cincinnati Children’s)
“This technology would make it possible to predict the eventual cardiac phenotype in pediatric patients and help guide their treatment and future monitoring,” said Jeffery Molkentin, PhD, lead author and a researcher in the Division of Molecular Cardiovascular Biology at Cincinnati Children’s and the Howard Hughes Medical Institute. “It could help when counseling patients about athletic endeavors, in which sudden death can occur with hypertrophic cardiomyopathy. Or it could help decide whether certain patients should consider an implantable cardioverter defibrillator to prevent sudden death as they grow into young adulthood.”
Inherited cardiomyopathy is a genetically diverse group of heart muscle diseases affecting about one of every 500 people. There are two primary clinical manifestations: hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). The diseases involve nearly 1,500 different gene mutations in sarcomeres, the part of the heart muscle that generates tension and contraction.
In HCM, the heart’s chambers and valves grow so that they are not symmetric. The dimension of the ventricular chamber is reduced, the interventricular septum thickens, and patients suffer from diastolic dysfunction (in which heart muscle doesn’t relax normally), causing an increased risk of sudden death from arrhythmia. With DCM, people have an enlarged left ventricular chamber accompanied by a lengthening of heart cells (myocytes) that results in reduced systolic function and eventually heart failure.
Effective drug regimens to manage the conditions do not exist, although there is research looking for new drugs. The only effective treatment at present is a heart transplant. This leaves an urgent need to develop new technologies to manage, treat, cure or prevent the diseases, according to researchers.
In developing the technology, scientists analyzed how sarcomeres generate tension coupled with alterations in calcium cycling, which is critical to heart function. The coupling of tension generation and calcium cycling is altered in patients with sarcomeric gene mutations. The alteration can be measured and then used to predict how the heart will change as disease progresses, Molkentin said.
To study the influence of gene mutations on this process, researchers tested an array of genetically altered mice. The mouse models were either normal (wild type) mice or those expressing different gene mutations for various cardiomyopathies.
This allowed researchers to examine tension generation and associated calcium cycling rates through heart muscle in a highly defined manner. That information was used to create a mathematical model for disease prediction that integrates the total tension generated by isolated cardiomyocytes. The tension-integrated, algorithm-based model was able to predict if hearts in mouse models would undergo hypertrophic or dilated cardiac growth.
The scientists next tested the computational model on human cells from cardiomyopathy patients by using induced pluripotent stem cell (iPSC) technology. Reprogrammed and derived from actual patient skin fibroblast cells, iPSCs can become virtually any cell type in the human body and then be used for scientific investigation of disease properties.
Molkentin and his colleagues generated patient-specific cardiomyocytes – which under a microscope can actually be seen pulsating rhythmically similar to a beating heart. Patient-derived iPSCs also carry the same genetic makeup (including mutations) as the person donating original starter cells. In the study, iPSC heart cells developed the same cardiomyopathy tension deficits as the patient’s own hearts.
Researchers then used their heart defect prediction method to see how accurately it determined the heart defect type of specific cardiomyopathy patients. With collaborators at the Stanford University School of Medicine, Molkentin and his colleagues generated four lines of developing, early-stage patient-specific iPSC heart cells (cardiomyocytes). They report that their technology accurately determined the HCM vs. DCM heart defect of the donor patients.
Researchers continue to develop and test the technology by using it to determine the cardiac disease state of patients with specific mutations in a sarcomere encoding gene. They caution the technology is years away from potential clinical use, pending further testing and refinement.




