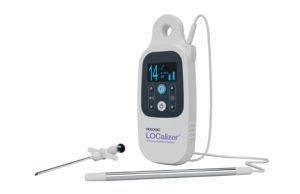
 Hologic (NSDQ:HOLX) said that its LOCalizer wireless radio frequency identification (RFID) breast lesion localization system has gained the CE mark. This system is designed for precise marking and targeting of lesions for breast-conserving surgery guidance, according to the Marlborough, Mass.-based company.
Hologic (NSDQ:HOLX) said that its LOCalizer wireless radio frequency identification (RFID) breast lesion localization system has gained the CE mark. This system is designed for precise marking and targeting of lesions for breast-conserving surgery guidance, according to the Marlborough, Mass.-based company.
Surgeons may implant the LOCalizer tag to replace the traditional wire-guided localization method to provide increased comfort and convenience for patients and their healthcare teams. Following placement, the miniature tag can be detected by a portable, handheld reader that indicates the location and distance in millimeters to the lesion, enabling the surgeon to pinpoint the correct area of breast tissue for removal. The tag can be implanted up to 30 days prior to breast-conserving surgery.
Hologic is displaying the system at the European Congress of Radiology meeting in Vienna, Austria through March 3. The LOCalizer system is manufactured by Health Beacons (Concord, Mass.) and is exclusively distributed by Hologic.




