“These developments support our plans to broaden distribution of the Macy Catheter and our continued commitment to quality,” said Igal Ladabaum, CEO at Hospi.
Hospi Corporation, a patient-centric medical device company, today announced that it has received CE Mark approval— Conformité Européenne, which is required for sale of medical products in the EU and other international countries—for the company’s flagship product, the Macy Catheter. Hospi also received ISO 13485 Certification, demonstrating that its quality systems conform to world-class standards.
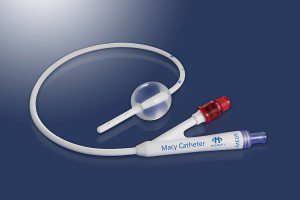
The innovative Macy Catheter is the only device designed solely for ongoing rectal delivery of medications and fluids. It offers clinicians and caregivers an easy, discreet, and comfortable alternative to oral or intravenous (IV) administration in a variety of settings when patients are unable to swallow. The Macy Catheter received Food and Drug Administration (FDA) clearance in 2014 and is being used by hospice and palliative care organizations throughout the United States. More recently, published research has demonstrated that the Macy Catheter can be effectively used in the emergency room and intensive care unit settings.
Hospi Corporation
www.hospicorp.com




