Top left/bottom: Johns Hopkins researchers use 3D printers to build co-venter design. Top right: Prisma’s VESper splitter device.
Amid the shortage of ventilators due to the COVID-19 pandemic, researchers are finding success using 3D printing to enable “ventilator splitting.”
The search continues to go on for more machines, but there have already been government actions taken to increase amounts, while researchers have taken it into their own hands to create homemade-style ventilators. However, there could be another option picking up steam.
A team led by engineers at Johns Hopkins University is developing and prototyping a 3D-printed splitter that would allow a single ventilator machine to treat multiple patients, according to an article on the university’s website.
“There is an emphasis right now on using engineering to develop open-source solutions to many aspects of the COVID-19 crisis, but especially for ventilator design and production,” Johns Hopkins Whiting School of Engineering assistant professor & team leader Sung Hoon Kang said in the article. “One approach is to use one ventilator to treat multiple patients. While this is feasible, it must be safe for all the patients. That means ensuring that each patient gets the care they need, without shortchanging anyone. This is what we set out to create.”
This innovation, sometimes called co-venting, is proving controversial, as guidance from the U.S. Dept. of Health and Human Services said it should be performed for the briefest time required with rapid transition to 1:1 patient-ventilator support when additional ventilators become available. However, the team at Johns Hopkins claims it can address the concerns over the safety of co-venting.
Medical professionals have expressed concerns that connecting several patients to one ventilator could spread germs and lead to cross-contamination, while there is also a school of thought in which ventilators shared by multiple people would not give them the necessary level of oxygen.
Kang said the Johns Hopkins design includes an airflow controller and flow meters to monitor and adjust airflow for each patient, while a filter designed to prevent cross-contamination is being added as well. The team has a prototype and plans to test the design on model lungs within weeks. Should the design be up to standards and approved by the FDA, the team plans to publish its open-source design for others to use via 3D printing.
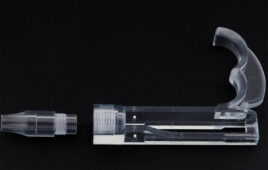
Meanwhile, Prisma Health received emergency use authorization (EUA) from the FDA for its VESper expansion device which is produced using 3D printing technology and designed to allow one ventilator to support up to four patients at once.
Greenville, S.C.-based Prisma developed the device design with material already in use for medical devices, and it’s possible to produce it at a minimal cost, according to a news release. The company is working with COVID-19 teams who can initiate emergency use of the prototype and will monitor clinical outcomes to see if performance is in line with FDA guidelines.
The VESper device is designed as a “Y” splitter tubing that can be easily produced, allow for the appropriate filtering of bacteria and viruses in the ventilator tubing, be impact-resistant and not impact the care of the other patients connected to the same machine. After testing the device with medical manikins, Prisma determined that it was capable of delivering the proper breathing parameters and could be brought into hospitals with FDA authorization.
“When we see rapid increases in patients who require machine-assisted breathing, an acute shortage of necessary equipment can happen overnight,” Prisma Health-Upstate chair of the department of medicine Dr. Peter Tilkemeier said in a news release. “The VESper device can be lifesaving when the number of critically ill patients requiring breathing support is greater than the number of available ventilators. A number of U.S. hospitals are likely to begin experiencing this with COVID-19.”