The next generation of intelligent balloon catheters depends on novel designs of ball-screw-driven pumps.
Bruce Gretz, Steinmeyer

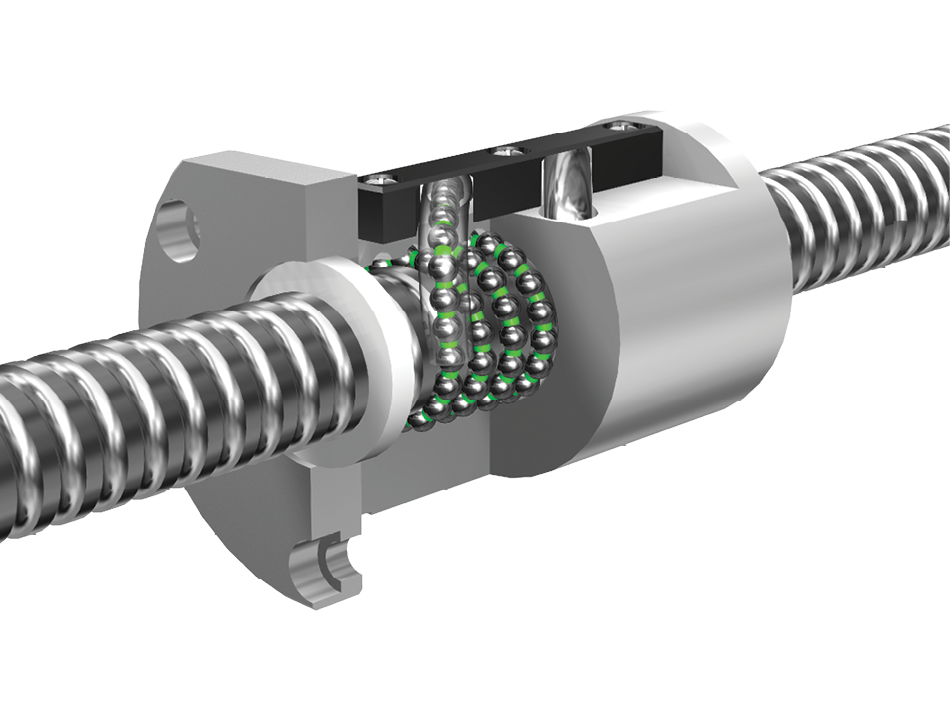
Miniature ball screw made by Steinmeyer. [Image from Steinmeyer]
More recent developments have focused on fully implantable ambulatory pumps serving as both the bridge-to-transplant and destination therapies for patients. Among the first was HeartMate, developed by Thoratec and now owned by Abbott. The first generation, which received FDA approval in 1994, used a pulsatile pump to mimic natural heart function. Next-generation devices — such as HeartMate II (2008), Impella (2008) by Abiomed, and HeartWare (2009) by Medtronic — use continuous-flow pumps with rotors. These designs have enabled smaller size with higher durability and reliability.
Fully implantable devices are very expensive to develop and produce and are limited to patients who can tolerate the highly invasive procedure. A key development in cardiovascular assist devices would be to combine the less-invasive approach of implantable balloons and external pumps with the high portability and reliability of fully implantable systems. This advance would allow the treatment of a much wider range of patients and conditions.
One of the main challenges to this approach is designing a wearable pump that can carefully control balloon pressurization. The pump must meet a demanding combination of performance requirements, including:
- Precise control of pressure;
- Reversible pressurization;
- Low weight;
- Compact size;
- Quiet action;
- Continuous operation for at least one year.
Mechanical engineers working on this designing such a pump faced a challenge. They built and tested early prototypes using traditional designs such as diaphragm pumps, which required both positive and negative pressure tanks. The resulting systems were far too large and heavy.
Their main innovation was selecting a precision ball screw as the drive mechanism and pairing it with a custom-engineered metal bellows as the pressure chamber. A ball screw delivers exceptional thrust density while enabling high linear acceleration in both the forward and reverse directions. Surrounded by the bellows, this results in an extremely compact and quiet design.
Ball screws are known for their high efficiency (>90%), smooth operation, and long life. Their efficiency enables the use of small motors with low power consumption, which in turn reduce the size and weight of the entire system.
A system that incorporates Steinmeyer ball screws targets intra-aortic applications and is currently in clinical trials. The pump’s final version will include ball screws produced with a proprietary super-finishing process called Optislite to ensure the quietest possible operation. This process removes the largest irregularities left over from thread grinding of the shaft. The resulting surface yields a noticeably smoother and quieter operation. Friction torque is also lowered and made more consistent, resulting in enhanced servo performance. In most applications that employ Optislite, product lifetime should be increased.
In future applications, balloon catheters will incorporate intelligence. These devices will use flexible electronics along the catheter for sensing, connectivity, and therapeutics. Ball screw-driven pumps will be ideal for supporting these future systems as well.
Bruce Gretz is EVP of Steinmeyer (Woburn, Mass.), joining the company in 2015 as national key account manager. He previously held sales and business development roles with several industrial firms in machine alignment, precision ceramics, and plant floor data acquisition. He holds an MBA from The Wharton School at the University of Pennsylvania and an M.S. in aerospace engineering from Stanford University.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.