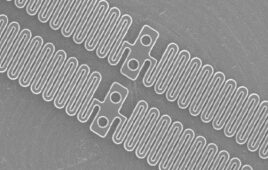
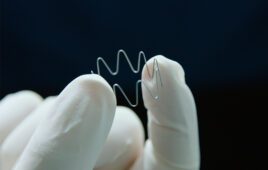
An ingestible LED like this could be swallowed to expose light-sensitive medical devices to light, helping them to break down in the body. [Image from MIT]
Medical devices can be inserted in the gastrointestinal tract to treat, diagnose or monitor a number of GI disorders. However, the devices have to removed through endoscopic surgery once completed.
The MIT researchers developed a light-sensitive hydrogel approach that can be incorporated into the medical devices to avoid endoscopic procedures.
“We are developing a set of systems that can reside in the gastrointestinal tract, and as part of that, we’re looking to develop different ways in which we can trigger the disassembly of devices in the GI tract without the requirement for a major procedure,” senior author of the study Giovanni Traverso said in a news release.
The researchers tested the device in pigs and found that the hydrogel-based devices could be triggered to break down when exposed to blue or ultraviolet light from a small LED.
“Given our interests in developing systems that can reside for prolonged periods in the gastrointestinal tract, we continue to investigate a range of approaches to facilitate the removal of these systems in the setting of adverse reaction or when they are no longer needed,” Traverso said. “We’re really looking at different triggers and how they perform and whether we can apply them to different settings.”
One advantage of using the light-based trigger is that light can work at a distance and doesn’t need to come into direct contact with the material it needs to break down, according to the researchers. Light also doesn’t naturally get into the GI tract, so the device can’t accidentally trigger.
To make the new material, the researchers designed a light-sensitive polymer gel that included a chemical bond that can be broken down when exposed to a wavelength of light between 405 and 365 nanometers (blue to ultraviolet). It also has stronger components like polyacrylamide that makes the material more durable but can still be broken down when exposed to light. The researchers also made the material as a double network, which means one polymer network surrounds another.
“You’re forming one polymer network and then forming another polymer network around it so it’s really entangled. That makes it very tough and stretchy,” lead author on the study Ritu Raman said.
The researchers can change the properties of the material by varying the composition of the gel. If there is more light-sensitive material in the device, it will breakdown faster but will be mechanically weaker. The researchers say they can also control how long it takes LED light to break down the material by using different wavelengths of light. Blue light works slower than ultraviolet light but has less risk to cells that are typically sensitive to damage from ultraviolet light.
All aspects of the material are biocompatible and the gel can be molded into a variety of shapes. The researchers have tested it as a seal for a bariatric balloon and an esophageal stent.
On the bariatric balloon, the balloon can be deflated by exposing the seal to a small LED light that can be swallowed and pass out of the body naturally. The balloon is made of latex and filled with sodium polyacrylate, which can absorb water. When tested in pigs, the balloons swelled up once placed in the stomach. A small, ingestible LED with blue light was placed in the stomach for six hours and the balloon slowly deflated, according to the researchers. When exposed to a higher-powered light, the material broke down in 30 minutes.
The researchers also tested the material in an esophageal stent which was able to be broken down and passed through the digestive tract when it was no longer needed.
The MIT researchers suggest the approach could be used to develop other degradable devices.
“This study is a proof of concept that we can create this kind of material, and now we’re thinking about what are the best applications for it,” Traverso said.
The research was published in the journal Science Advances and was funded by the National Institutes of Health, the Bill & Melinda Gates Foundation, the Koch Institute Support Grant from the National Cancer Institute and an AAAS L’Oréal USA for Women in Science Fellowship.