Any company designing a medical device today needs to take connectivity into account. The days of isolated medical data tied together in a patchwork of paper, photos and electronic means is coming to an end.
Bill Betten, Betten Systems Solutions

Typical connected device block diagram. [Image courtesy of Betten Systems Solutions]
While much of medical device design is consistent between a traditional device and a connected one, additional requirements must be considered early in the design to ensure a smooth development. The critical design considerations include:
• Sensor type – What data do you want to collect?
• Body location – External or implanted, where on the body?
• Frequency of data acquisition – Continuous or periodic?
• Frequency of data transmission – Continuous, hourly, daily, etc.?
• Architecture – Local processing, distributed or cloud?
• Connectivity – Wired or wireless; WiFi, cellular or Bluetooth?
• Power – AC or battery (rechargeable or not)?
• Communication protocols and standards.
• User experience – Who are the users? Look and feel; Displays
• Security – Data locations; Connection points; Physical security
• Regulatory – FDA; FCC; UL, CE, radio licensing by country, etc.
Space doesn’t permit a detailed discussion of each of these, but they are all linked together and thus need to be considered from a system perspective. Typical connection methods and their pros and cons are discussed in the chart. Of particular importance are the periodicity of data collection and its transmission, as well as distance from the sensing device to the gateway. These considerations drive many of the other design elements, including size, power and storage. Devices inside the body also pose a different challenge due to the absorption of the signal by body tissue.
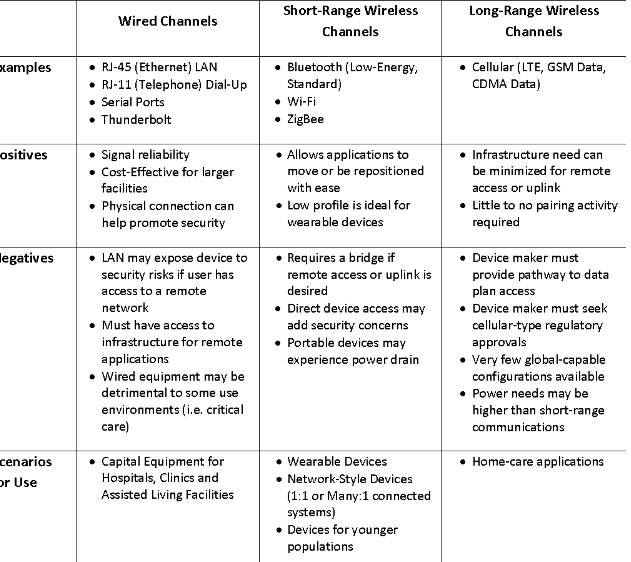
Comparison of typical device connection schemes [Image courtesy of Betten Systems Solutions]
Patients will benefit from improved health outcomes and quality of life, real-time support and interventions, the extension of care at home after discharge, and reduced hospital stays. Providers will benefit through the extension of clinical environments into a patient’s home, increased frequency and accuracy of patient health data, and the ability to continue monitoring patient health, particularly for chronic cases. The result should be reduced costs from re-admissions and reduced hospital stays. Payers will have a better view of patient compliance practices and more accountability from patients, which should result in lower costs. Finally, connected devices enable medical service in remote environments.
The fusion of objective, reliable and timely patient information may truly usher in the era of “big data,” revolutionizing the assessment and delivery of health care. While we may not yet quite have developed the Star Trek tricorder, we certainly have come a long way from the two-way wrist communicator of Dick Tracy in the ’40s. The technology is no longer a gating item to the potential reform of our medical health care delivery system, and hopefully, the payment and delivery system will evolve to fully utilize those capabilities.
Bill Betten is the president of Betten Systems Solutions, a product development realization consulting organization. Betten uses his years of experience in the medical industry to advance device product developments into the medical and life sciences industries, helping clients to develop innovative medical devices and adapt to a changing environment. He most recently served as director of business solutions for Devicix/Nortech Systems, a contract design and manufacturing firm.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.




