When designing a co-extruded multi-layer tube for an intravascular application, the final physical properties of the polymers used are not the only factor. For optimal extrusion, it is also important to consider the effects of the viscosity, the polymers’ melt temperatures, and their placement in the structure.
Steve Maxson, Spectrum Plastics Group
[Image courtesy of Spectrum Plastics Group]
So what does a medical device creator need to know when it comes to multi-layer coextrusion of dissimilar and similar polymers?
Co-extruding dissimilar polymers
Multilayer coextrusion poses some challenges, particularly in creating uniform wall thickness. Differences in the viscosities, velocities and melt temperatures of dissimilar polymers may cause problems, including delamination.
Dissimilar polymers have different chemistries with low levels of interlayer adhesion and are subject to interfacial instability. This is compounded when polymers with low surface energies are used in the multi-layer structures. As a result of the chemical incompatibility, these different grades of polymers do not form strong bonds with each other, even upon coextrusion. Tubing made of layers of dissimilar materials tends to delaminate.
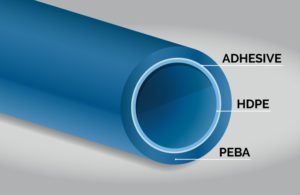
Medical device and co-extrusion polymer selection
Multi-layer tubing suitable for percutaneous transluminal catheters used to deliver an angioplasty balloon may require an inner layer of a lubricious polymer such as HDPE to enable the catheter to advance over the guidewire. The adhesive middle layer could be made of a modified LLDPE, and the outside may be a soft, “bondable” layer such as PEBA for bonding a PA12 angioplasty balloon to the catheter shaft.
Using adhesive to bond the balloon to the catheter shaft is not optimal because the adhesive may fail, and because the adhesive adds to the overall profile of the catheter. Percutaneous transluminal catheter tubing contains very thin wall thickness down to 25 microns with internal diameters designed to support 0.014-in., 0.018-in. and 0.035-in. delivery platforms.
Viscosity and velocity
From an extrusion standpoint, viscosity is the most important flow property in multi-layer polymer selection. Typically, the inner layer has the highest viscosity, and the outer layer has the lowest viscosity, as the low-viscosity melt can encapsulate the high-viscosity melt while flowing through the die head and tooling channels.
Polymers with compatible glass transition temperatures (Tg) and melt temperatures (Tm) should also be chosen for consistent layer distribution. The glass transition temperature of each polymer layer should be between 85% and 115% of the glass transition temperature of the adjacent layer polymer(s).
Multi-layer tubing die flow channels are typically designed to provide uniform velocity of each polymer flow at the die exit, assuming uniform incoming melt temperatures. When a higher-velocity polymer layer merges with a lower-velocity layer, the high-velocity layer slows down, causing wave-like flow instabilities at the interface. Interfacial instabilities result in intermixing of the layers, decreased performance or delamination, and poor aesthetics in catheter tubing.
Non-uniform melt temperature also affects polymer velocity going into and through the die. In a multi-layer coextrusion process, such melt temperature gradients can indiscriminately affect individual layer uniformities. Extrusion processing conditions can be optimized to avoid the occurrence of interfacial instabilities by controlling the melt temperatures and thereby the velocity ratios of each melt stream.
Adhesive (tie) layers used in multi-layer co-extrusion of dissimilar polymers
An adhesive polymer acts as a bonding layer and a stress reliever at the interface of hard-to-bond polymers. It promotes uniform wall thickness and discourages delamination during co-extrusion as well as under normal and extreme operating conditions, such as high balloon pressures.
Co-extruding similar polymers
High-pressure balloon tubing may have a wall with at least three adjacent coextruded layers of similar polymers such as PA12, with varying durometer ranges for each layer. After the tri-layer tubing or preform is reheated and formed into a balloon, the non-compliant, tri-layer dilatation or stent delivery balloon can have a burst pressure of at least 30atm (440psi).
Dual or tri-layer balloon tubing has structural integrity and reduces the standard deviation significantly from balloon to balloon when completing burst performance. The reduction in standard deviation allows the balloon to be rated at a higher pressure. A defect in one layer of a tri-layer tube made of the same material doesn’t affect the second or third layer or vice versa.
Steve Maxson is VP of sales, vascular technologies for Spectrum Plastics Group. The company is a global manufacturer of medical polymer components and sub-assemblies, including multi-layer catheter tubing and thin-walled, gel-free balloon tubing.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.




