More than 2.5 million people around the world are undergoing dialysis (hemodialysis) for end-stage renal disease, according to the European Renal Care Provider Association.
The U.S. Food and Drug Administration (FDA) has granted Medtronic investigational device exemption (IDE) for the IN.PACT Admiral drug-coated balloon (DCB) for patients with end-stage renal disease.
The IDE allows Medtronic to begin a randomized study for the device that will evaluate it as a treatment compared to balloon angioplasty, for failing arteriovenous (AV).
Dialysis takes place in AV access sites.
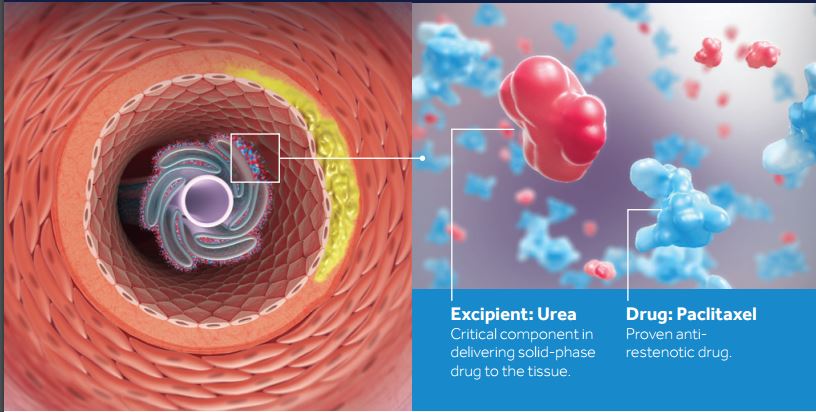
(Credit: Medtronic)
“Over time, thickening of the vessel walls limits the ability to use a dialysis access site, requiring repeat interventions, which increase health care utilization and reduce quality of care. Repeated procedures are also associated with high technical failure rates and reduced quality of life for patients,” according to Medtronic.
“In the past, when the AV access site became narrowed, the only option was use of a standard percutaneous transluminal angioplasty (PTA) balloon to re-open and regain access for dialysis. This would often result in restenosis and high rates of re-intervention,” says Andrew Holden, M.D., director of interventional radiology at Auckland Hospital and associate professor of radiology at Auckland University. “Patients on dialysis need alternatives to help reduce and manage stenosis of their AV access sites. It is important to effectively evaluate options such as this DCB, which already has clinical evidence in patients with peripheral artery disease (PAD) in the upper leg. “
The two-year study will include about 330 patients and take place in about 30 sites in the United States, Japan, and New Zealand.
“IN.PACT Admiral DCB has demonstrated superior clinical outcomes in patients with PAD in the upper leg. Through our IN.PACT clinical program, we are looking at ways this DCB technology can address challenging lesions, and we have specifically designed the DCB with extended sizes for use in AV access,” says Mark Pacyna, general manager of the Peripheral business, which is part of the Aortic & Peripheral Vascular division at Medtronic.
“Following receipt of the CE Mark for this indication last year, the IDE approval and study initiation reflects our commitment to innovation and, most importantly, to our patients.”




