Innovation in silicone technology has moved beyond defining specific material characteristics and into offering implantable devices that cure and form into shape inside the human body.
Benny David, NuSil from Avantor
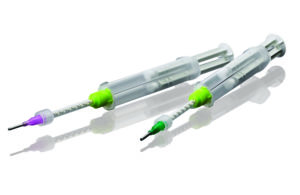
Designed to allow sterilization of uncured medical-grade silicones, this packaging for in situ curable silicones features a dual-cartridge prefilled dispensing system. (Image courtesy of NuSil by Avantor)
Medical device manufacturers have long relied on highly purified silicone elastomers to fabricate components or entire devices for implantation. Now, recent innovations offer manufacturers new opportunities to create in situ-cured implants that are highly customized and require less invasive implantation procedures.
Silicone has a long-established history of biocompatibility, making it a highly valued material among device makers. Its unique chemical make-up allows silicone manufacturers to precisely fine-tune the material at the polymer level to meet specific medical device requirements, such as cured physical properties ranging from extremely pliable to very rigid and uncured characteristics from pourable to thixotropic.
With qualities like biocompatibility and versatility, silicone has historically been used for a range of applications, from cardiovascular pacemakers and Cochlear implants to implantable infusion pumps, intraocular lenses and drug delivery implants. It’s attractive for a variety of medical uses, like high-precision molded parts, lubricious coatings for medical devices, soft silicones for wound care and soft tissue implants.
Silicone elastomers are typically used to fabricate components of devices, or entire devices, which are then assembled, packaged and sterilized prior to surgical implantation. Now, innovation in silicone technology has moved beyond defining specific material characteristics and into offering implantable devices that cure and form into shape inside the human body. In essence, this creates personalized, “real-time” implants that conform to the shape at the implant site, rather than requiring the body to conform to a standard implant shape.
The traditional method for producing silicone implant devices can vary between manufacturers. Since common silicone sterilization methods, like gamma and electron-beam irradiation or autoclave, can affect the characteristics or workability of the silicone, ethylene oxide (EtO) is often used for medical device sterilization. The cured component is then combined with other device components, placed in a single package or tray, which is then sealed and sterilized prior to surgical implantation.
How in situ cure technology works
New silicone dispensing systems developed specifically for the healthcare industry allow in situ sterilization of uncured medical-grade silicones. Featuring a pre-filled, dual-cartridge dispensing system, each barrel employs a gas-permeable plunger seal that allows an EtO sterilant gas to permeate the plunger seal and sterilize the uncured silicone inside the cartridge. The silicone can then be delivered directly into the human body wherever the device is needed, whether it’s intended to provide cushioning or fill a void.
Tests to verify sterilization have demonstrated:
- Effective sterilization.
- No residual EtO post-sterilization.
- Minimal change to key silicone properties, such as rheology, durometer, modulus, work time and cure rate.
The advantages of in situ cure technology
In situ curing gives device makers a novel therapeutic solution: a customized, “real-time” implant. From elasticity to fatigue resistance to hardness, manufacturers can target specs for specific properties that increase a device’s functionality.
This versatility creates a range of opportunities for new therapies in cardiovascular, neurological, urological and ophthalmic aesthetic implants and drug delivery applications. Research and some development are already underway in many of these applications. It’s possible in bone and vertebral repair, for example, that in situ-cured silicones could enable a custom-fit device that is more flexible than current offerings, thus allowing for a greater range of motion for the patient.
The ability to specify the material’s final physical properties is particularly important when designing devices for implantation. For example, specifying a hardness level can offer firmer support or softer cushioning. Likewise, viscosity can be optimized for the desired implant’s location within the human body. Cure time can be adjusted to the device manufacturer’s specification so that the silicones cure at body temperatures in the time frame desired. The material can also be made radiopaque, and either insulative or conductive properties can be tailored within the silicone formulation.
The dispensing system innovation enables new therapies to be developed — ones that could not be achieved before because of the surrounding anatomy. By providing an alternate method of surgical implantation, in situ-cured silicone can be provided as a part of the surgical kit and formed within the body to create a custom-fit device. As a result, therapies can be customized to the patient’s anatomy.
Understanding in situ curing technology and its advantages allows medical device makers to explore opportunities to fabricate implanted silicone-based devices that are customized for fit and performance and require less-invasive surgical procedures, resulting in shorter recovery time for the patient.
Benny David is business development director for biomaterials and drug delivery for NuSil. He spent his career focused on the polymer industry in R&D, business development and commercial roles in the healthcare, aerospace, defense, electronics, industrial and automotive industries.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.



