Inspire therapy works inside the body and with the patient’s natural breathing process to treat sleep apnea. It continuously monitors breathing patterns while the patient sleeps. Based on the patient’s unique breathing patterns, the system delivers mild stimulation to key airway muscles, which keeps the airway open.
Furthering obstructive-sleep-apnea (OSA) research, Inspire Medical Systems announce the acceptance for the publication of its manuscript, “Upper Airway Stimulation for Obstructive Sleep Apnea: Durability of the Treatment Effect at 18 months” in SLEEP, the official journal of the American Academy of Sleep Medicine, on June 4, 2015.
Durability data from Inspire Medical’s Upper Airway Stimulation therapy demonstrates significant reductions in OSA severity, while maintaining quality of life measures at the 18-month mark. The outcome data from the 126 patients who underwent the Stimulation Therapy for Apnea Reduction (STAR) Trial demonstrates:
- A 67.4% reduction of apnea-hypopnea index at 18 months.
- Quality of life measures including daytime sleepiness and functioning showed clinically meaningful improvement over baseline.
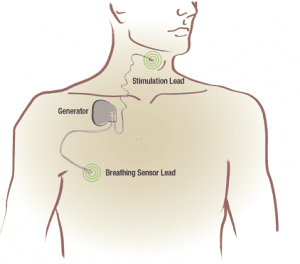
Inspire therapy is an FDA-approved treatment for people with moderate to severe OSA who are unable to use or do not get relief from Continuous Positive Airway Pressure (CPAP). In contrast to CPAP, Inspire therapy works inside the body and with a patient’s natural breathing process. The system includes a small neurostimulator, a sensing lead and a stimulation lead. The device is turned on with a handheld sleep remote and, when activated, the device senses breathing patterns and delivers mild stimulation to key airway muscles which keeps the airway open during sleep.

The Inspire system consists of four components: a small generator, a breathing sensor lead and a stimulation lead—all controlled by the small handheld Inspire sleep remote. The user simply turns the therapy on at night before bed and off in the morning when he or she wakes up.
“The clinical evidence supporting the use of Inspire therapy is quite strong,” said Tim Herbert, CEO of Inspire Medical Systems. “With the publication of the 18-month durability data, there are now nine peer-reviewed publications covering the safety, effectiveness and durability of Inspire therapy. We also expect publication of the two-year and three-year outcomes data in the second half of 2015.”
In addition, two-year and three-year objective and subjective outcomes data from the STAR Trial will be featured during several scientific sessions at the American Academy of Sleep Medicine’s SLEEP 2015 meeting in Seattle, June 6-10, 2015. SLEEP attendees can learn more about Inspire therapy and the recent research results during the following conference presentations in Seattle:
- Tuesday, June 9:
- S09: “Randomized Trials of Surgery for OSA”
- Room 6C; 10:20 a.m. – 12:20 p.m.
- O19: “Obstructive Sleep Apnea Treatment”
- Room 6B; 4:30 p.m. – 4:45 p.m.
- S09: “Randomized Trials of Surgery for OSA”
- Wednesday, June 10:
- D04: “How Do New Clinical and Consumer-Oriented Tools Fit Within the Practice of Sleep Medicine?”
- Room 6B: 8:00 a.m. – 10:00 a.m.
- D04: “How Do New Clinical and Consumer-Oriented Tools Fit Within the Practice of Sleep Medicine?”
Inspire Medical Systems
www.inspiresleep.com




