The company’s system is accurate, consistent and reliable for balloon inspection processes. Furthermore, the Auto-i 360 significantly reduces inspector-to-inspector variability.
Interface Catheter Solutions recently announced that its new Auto-i 360 balloon visual and dimensional inspection system upgrades significantly reduce cycle times. Interface completed recent Gage R&R (repeatability and reproducibility) analyses of the Auto-i 360. Results show that the system is accurate, consistent and reliable for balloon inspection processes. Furthermore, the Auto-i 360 significantly reduces inspector-to-inspector variability.
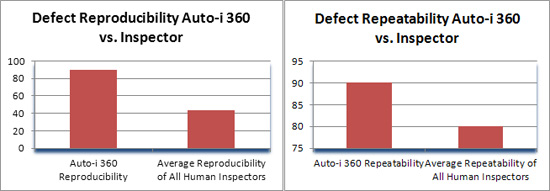
The visual inspection Gage R&R results show that the Auto-i 360 is capable of tabulating and analyzing the same defect multiple times analyzed by a single trained inspection operator. This test was completed with two trials and two trained technicians looking for repeatability and agreement on the system results.
The overall average agreement on repeatability was 90% (Chart A) a figure deemed highly relevant within industry standards. Similar study results with trained quality inspectors and without using the Auto-i 360 resulted in higher variability and reduced agreement between inspectors. The Auto-i 360 is proven to be a highly reliable inspection tool with automatically tabulated reports traceable to production lot, inspector and balloon.
Defect reproducibility was also evaluated to see the same defect from operator to operator perspective on multiple trials. In this case, previous repeatability results were compared to determine Auto-i 360 capability. Results were also positive at 90% (Chart A) well within expectations. The Auto-i 360 can improve manufacturer efficiency and yield significant cost saving.
“Completing the Gage R&R for our own balloon manufacturing inspection and validation process was important not only for our needs and our customers stringent quality requirements,” said Nabil Jubran, director of quality and regulation at Interface Catheter Solutions. “With these results we are confident in the robust balloon inspection capabilities and effectiveness.”
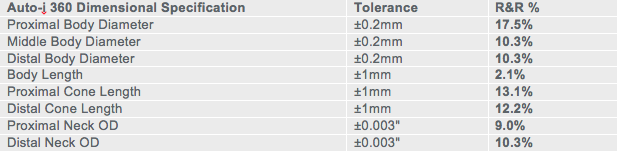
Dimensional capability Gage R&R also had positive results. Inspectors and operators input dimensional criteria into the system, including center, left and right body diameter; left and right-stem diameter and cone length; and body length. Dimensional results were measured against Interface balloon tolerance inspection criteria that are highly stringent in evaluating balloon quality. At less than 20%, the results were above industry standard in all measurement criteria (Chart B).
“The Auto-i 360 has been developed with customer participation including our own production and quality engineering staff to create a highly-effective inspection tool with specification criteria based on years of balloon inspection experience,” said David Yanes, director of equipment at Interface Catheter Solutions. “The Gage R&R results confirm the efficacy of the system and its ability to operate successfully within tight tolerance inspection standards. The Auto-i 360 significantly decreases inspection time reducing cost and improving yields.”
The is the only one that provides visual and flaw, and dimensional measurements of medical balloons in a single operation, tested and proven in one of the largest balloon-contract manufacturing facilities in the world.
Gage R&R data shows significant increases in consistency, accountability, and quality in the inspection process. Inspection includes classification and size of defects with pass or fail analysis based on user-selected criteria. The balloon-inspection system operates with a sophisticated vision system and analysis program with an intuitive user interface. The Interface inspection system provides immediate detailed visual and data reporting used in quality manufacturing inspection and research and development.
Interface Catheter Solutions
www.interfaceusa.com