KCI, an Acelity Company, today announced the U.S. launch of the ABTHERA ADVANCE Open Abdomen Dressing. ABTHERA ADVANCE Dressing is the next generation temporary abdominal closure device leveraging the technology and success of ABTHERA Open Abdomen Negative Pressure Therapy in bridging abdominal wall openings where primary closure is not possible and/or repeat abdominal entries are necessary. In a comparative study of healthy pigs with an open abdominal wound which were treated with either ABTHERA SENSAT.R.A.C. OA Dressing or ABTHERA ADVANCE Dressing at -125mmHg, the ABTHERA ADVANCE Dressing demonstrated significant increase in overall tissue, skin and fascia movement, with no change in intra-abdominal pressure.
“Developing and expanding solutions for surgical management is a key focus for us as we invest to broaden KCI’s industry leading NPWT portfolio,” says R. Andrew Eckert, president and CEO of Acelity. “The ABTHERA ADVANCE Open Abdomen Dressing is unique to the market, and we believe has tremendous potential for surgeons managing challenging open abdomens.”
(Image credit: KCI)
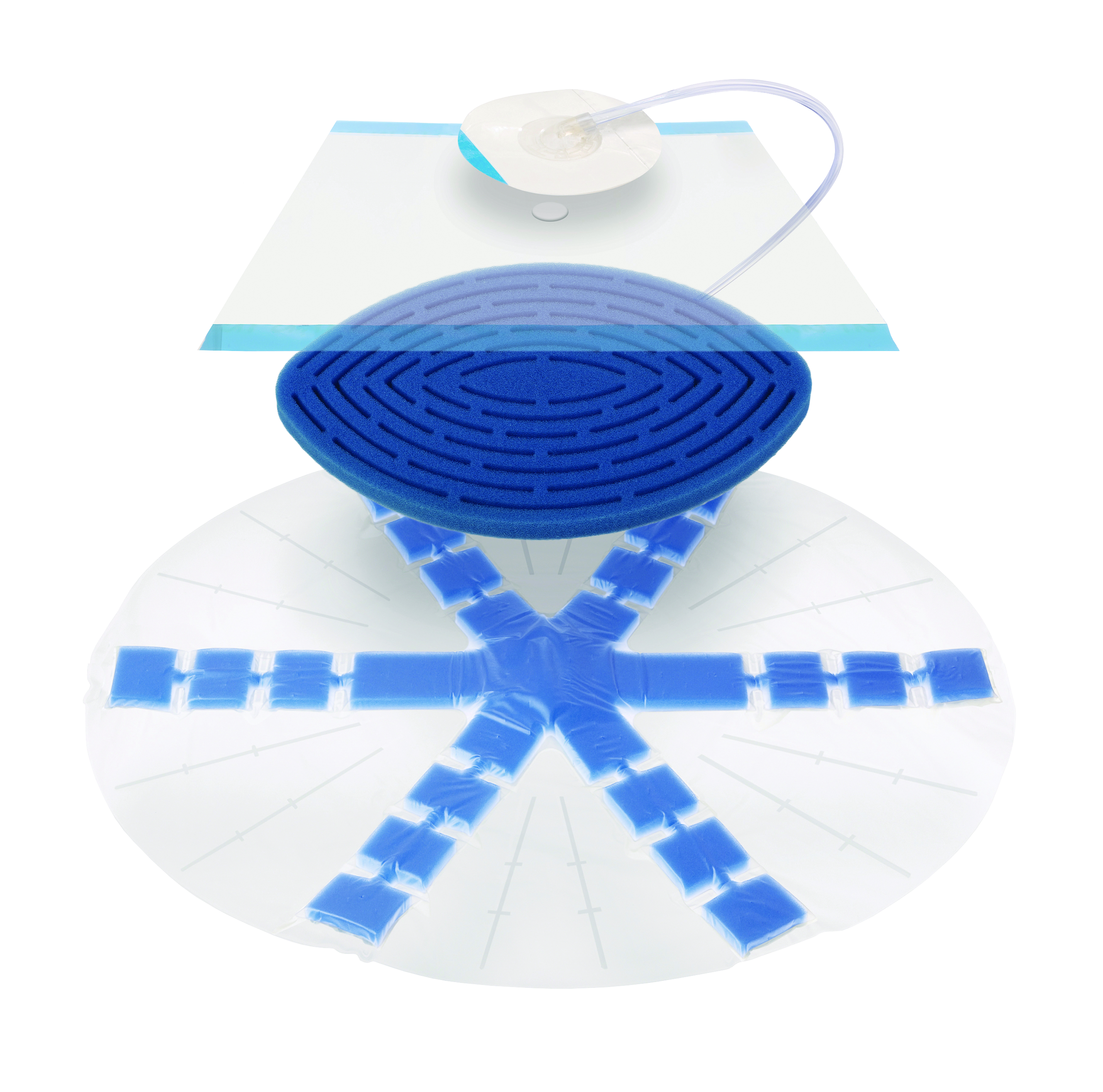
The ABTHERA ADVANCE Perforated Foam collapses medially while under negative pressure, actively drawing wound edges together. ABTHERA ADVANCE Dressing is designed to manage the open abdomen by:
- Actively removing fluid and reducing edema
- Providing medial tension which helps minimize fascial retraction and loss of domain
- Providing a separation between the abdominal wall and viscera
- Protecting abdominal contents from the external environment
- Allowing for rapid re-entry with no requirements for sutures for placement
The increasing numbers of septic and/or contaminated abdomens pose a difficult challenge to primary fascial closure. Clinical evidence demonstrates that ABTHERA Therapy is associated with improved clinical outcomes, such as increased patient survival, improved primary fascial closure rates, lower intensive care unit stays and more when compared to Barker’s vacuum-packing technique (BVPT). According to one open label, prospective observational study, ABTHERA Therapy patients were more likely to achieve primary fascial closure and had a reduction in 30-day all-cause mortality compared to patients who received BVPT. In a separate study, that was a randomized controlled trial, ABTHERA Therapy also had a significant reduction in 90-day all-cause mortality rate compared to BVPT.
“Leaving the abdomen open after surgery with temporary abdominal closure when managing complex abdominal problems is associated with complications such as fistula, infection and hernias – early primary fascial closure is critical in mitigating those risks,” says Mark Kaplan, MD, chairman of the division of trauma and surgical critical care and associate shairman pf the department of surgery at Albert Einstein Medical Center in Philadelphia. “I have seen the dramatic results ABTHERA Therapy can have for my patients and practice — especially those cases where achieving primary fascial closure is challenging and patients are at risk for long-term complications. I am excited about the preclinical evidence for the new ABTHERA ADVANCE Dressing and I look forward evaluating it in the coming months.”




