Traditionally, manufacturers of medical devices have been wary of designing in Lithium Ion batteries, mainly due to reasons of safety. Today’s Lithium Ion batteries are subject to different tests and testing standards, e.g. electrical safety, temperature test, shock and vibration tests in various environmental conditions (humidity, temperature, pressure), to avoid critical issues in daily use.
Every year new national mandatory approvals appear that must be carried out as a condition to entering local markets, which, in addition to the costs of tooling and development, can prove very expensive. So, understandably, using standard batteries with all mandatory approvals produced by an ISO13485 certified manufacturer, and without the additional incurred costs, makes life very easy.

LiIon Batteries (Credit: Michael Müller)
Battery Management (BMS)
To make Lithium Ion batteries safe and reliable, a state-of-the-art battery management system (BMS) is necessary. The BMS enables the battery to operate in a ‘smart’ manner, to perform cell balancing and to protect itself on two different levels. A smart battery monitors all internal values and can decide how to be treated from an external perspective. A BMS is equipped with self-protection features against over-voltage, overcurrent, short current, under-voltage and over-temperature. Different safety values for charge and discharge are defined in the controller. A high quality battery should also comply with the JEITA rules with adopted charge-voltage levels based on actual temperature.
All the values can be communicated via SMBus1 to a charger or the medical device. For example, the medical device can read the current state of charge and can calculate its remaining runtime. The cell balancing is able to control each cell in a series stack. For example, when a series battery is being charged with a smart charger, single cells can be by-passed when they become fully charged and all other cells are then charged until full. So, any imbalance in the cells is removed and the battery pack charging is optimized to ensure the batteries longest possible lifetime.
An SMBus battery communicates via a simple bidirectional serial data interface. A host processor uses the interface to access various battery pack registers. With SMBus communication, a battery can provide over 30 bits of information regarding its manufacturing data, status (state of charge, discharge, cycle life, temperature, etc.), access its registers and other data.
Block Diagram for a Smart Battery Pack
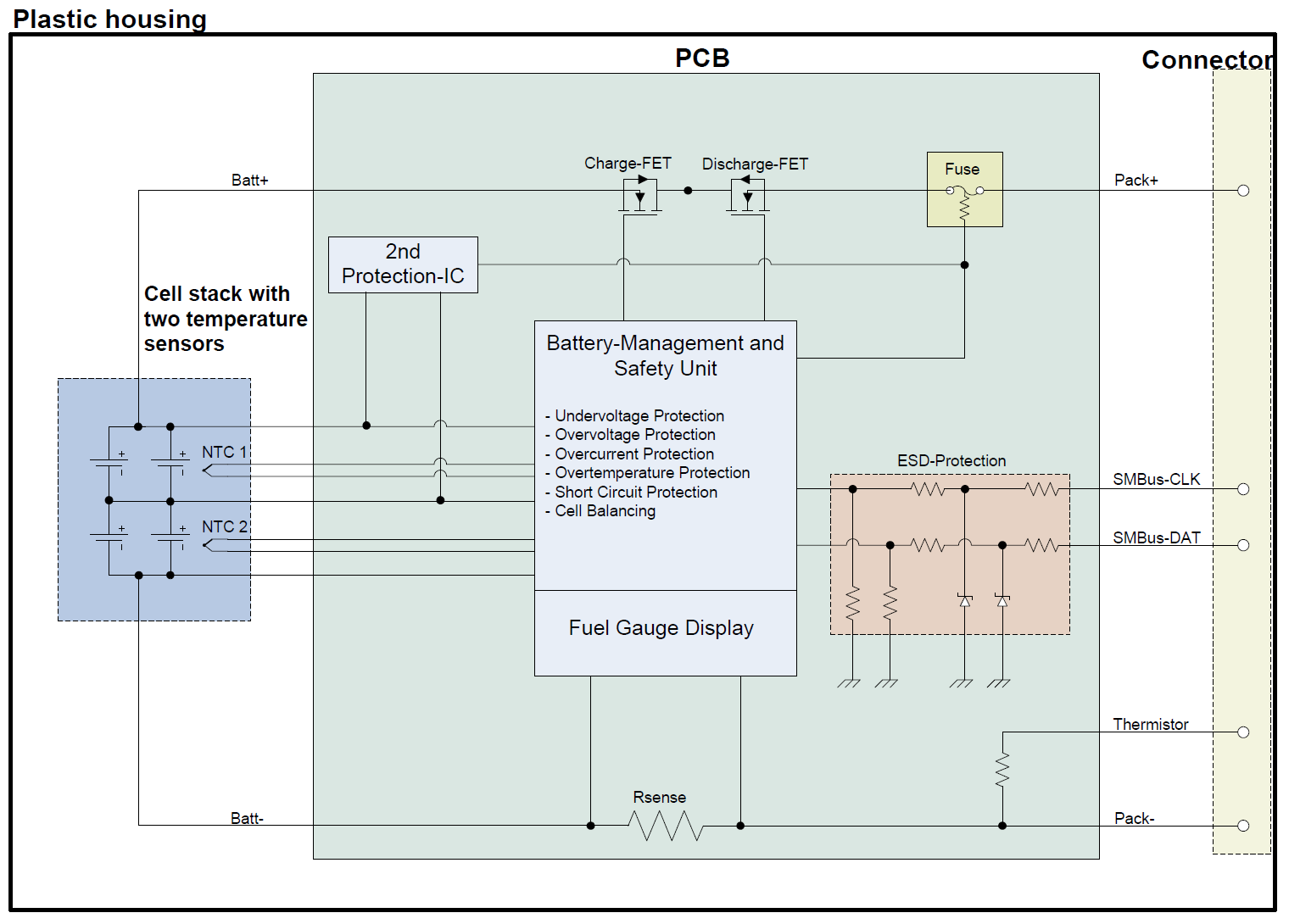
(Credit: Michael Müller)
Mandatory Approvals
First of all, a Lithium Ion battery has to fulfil the UN38.3 testing method for transport. Then the battery packs can be marked with the UN symbol and can be shipped without dangerous goods declaration, assuming it fulfils the limit of less than 100Wh.
Next, a CE declaration based on EMC measurements and to show the compliance with RoHS directive, REACH regulation and the Battery Directive, has to be done for the EU market. To implement the Lithium Ion battery into a medical device the medical standard IEC/EN60601-1 with Amendment 1 requires the certification of batteries to the latest revision of IEC/EN62133 (2nd edition), documented in a CB report. Based on this CB report the batteries have to be tested according to national mandatory standards.
Today we see mandatory standards as UL for USA and Canada, PSE for Japan, KC for Korea, RCM for Australia and New Zealand, EAC/Gost for the Russian Federation and BIS for India. In China, we have a voluntary CQC Mark, based on the mandatory GB31241 standard.

RRC2020 with approval marks (Credit: Michael Müller)
Security
To protect a medical device to be powered by a grey-market battery, a smart battery can be equipped with an authentication key based on the SHA-1 standard. This key can be a standard one for all batteries of the manufacturer or a custom specific for the medical device manufacturer. If a battery is put into the device, first it will read out the authentication key from the battery after the communication is established. If the key matches, the battery can be used in the device. Otherwise it will be rejected.
The custom specific authentication key can be combined with private labeling of the battery. In this case the medical device manufacturer can fully control the use of its battery as well as the complete aftermarket business for spare batteries.
Reference:
1: Refer to the following SMBus specifications:
- System Management Bus Specification (Rev 1.1, Dec 11, 1998)
- System Battery Data Specification (Rev 1.1, Dec 15, 1998)
- System Battery Charger Specification (Rev 1.0, Jun 27, 1996)
- Further information: www.SMBUS.org