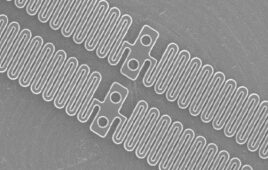
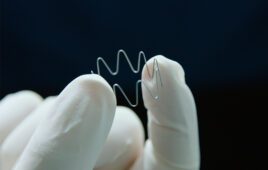
Liquid silicone rubber (LSR) is great for medical device applications, but is the tooling worth the trouble?
Dave Theiss, Robin Industries
Liquid silicone rubber (LSR) is a sought-after material for medical applications. But there are tooling challenges and expenses associated with LSR that need to be understood by medical manufacturers looking to use the material.What’s so great about LSR
Many LSR materials are biocompatible, and some grades are approved for implants. Devices made from LSR are temperature stable and can withstand harsh cleaning and disinfectants. Further, LSR resists discoloration from UV exposure, is scratch resistant, and retains a cosmetic attractiveness throughout the product lifecycle.
The material comes in an array of durometers, available at 5–90 durometer, that can be mixed to match any color. It can also be made optically clear, and it is second only to glass in light transmission.
LSR has a low viscosity that can be molded into components with thin walls or small features. Once cured, the material is flexible, and can be easily removed from a mold form, even with undercut shapes. Some grades of LSR materials will bond to specific substrates during overmolding, thus eliminating the prepping step required by other overmolded materials.
In addition, LSR parts can be fully cured in seconds rather than in minutes (compared with HCR silicone), which speeds up cycle times.
Trouble with tooling
LSR tooling is expensive, and building molds requires high precision and expertise beyond what’s needed for rubber and thermoplastics.
The liquidity of the material means that the cavity inserts must be absolutely precise to prevent flash extension. For example, tooling for many LSR processes will flash with fits as tight as 0.0002–0.0003 in.
Ejector pins can be used in LSR molds but they must have a tapered shutoff and can’t invade the parting-line shutoff area. Typically, ejector pins are avoided because any amount of debris or rubber that builds on the ejector valve seat will cause flash.
LSR molders will likely use a vacuum in most tooling to get rid of outgassing, because venting, which is used in other thermoplastic processes, doesn’t work as well. LSR comes in two parts and must go through a chemical curing process before being injected into the mold. Depending on the final part needs, venting might need to be ten-thousands-of-an-inch deep to control flash for tight specifications.
Curing LSR requires a precise hand. The molds are usually heated to 270-360 °F to cure and require electrical cartridge heaters strategically placed into the mold. Heating molds to these temperatures will start the cure process in seconds. LSR also begins to cure at room temperature once the reactive components are mixed. Therefore, the process requires flowing coolant to control the temperature up to the cavity.
Shutting down an LSR process is time-consuming and generates material waste. The dosing system requires disassembly and each component must be cleaned out with a solvent. The A and B hoses are removed and the static mixer completely disassembled and again, thoroughly cleaned with solvent. System clean-out can take up to 4 hours and disassemble and cleaning tools will take the rest of the workday.
Overcoming tooling issues
Molders with LSR expertise are aware of these challenges and can adjust by simplifying mold construction. Some use cavity inserts that go into standardized mold frames. These frames will have a hot or cold runner system that can adapt to the cavity inserts, thus eliminating the need to build a frame for each part.
Miniature material dosing systems can minimize set-up and material waste. Table-top dosing systems use short hoses or attach directly to the injection unit to simplify priming and cleanout process. A pressure pot is a sealed reservoir that uses air pressure to force the LSR into the injection unit. The pressure pot requires premixing the LSR as part of the set-up, but eliminates the need to prime and clean out static mixing units. Further, the pots are easily removed from the machine and can be refrigerated to extend the life of the pre-mixed LSR.
These adaptations will not cut out all costs, but they do simplify set-up and shutdown procedures Because of the need for a high level of precision, and the risks of waste, expenses can add up quickly. It is important to work with a molding house that has LSR experience and can offer part design consulting early in the process.
Dave Theiss is technical director at Robin Industries (Independence, Ohio).
(See the best minds in medtech live at DeviceTalks West, Dec. 11–12 in Orange County, Calif.)