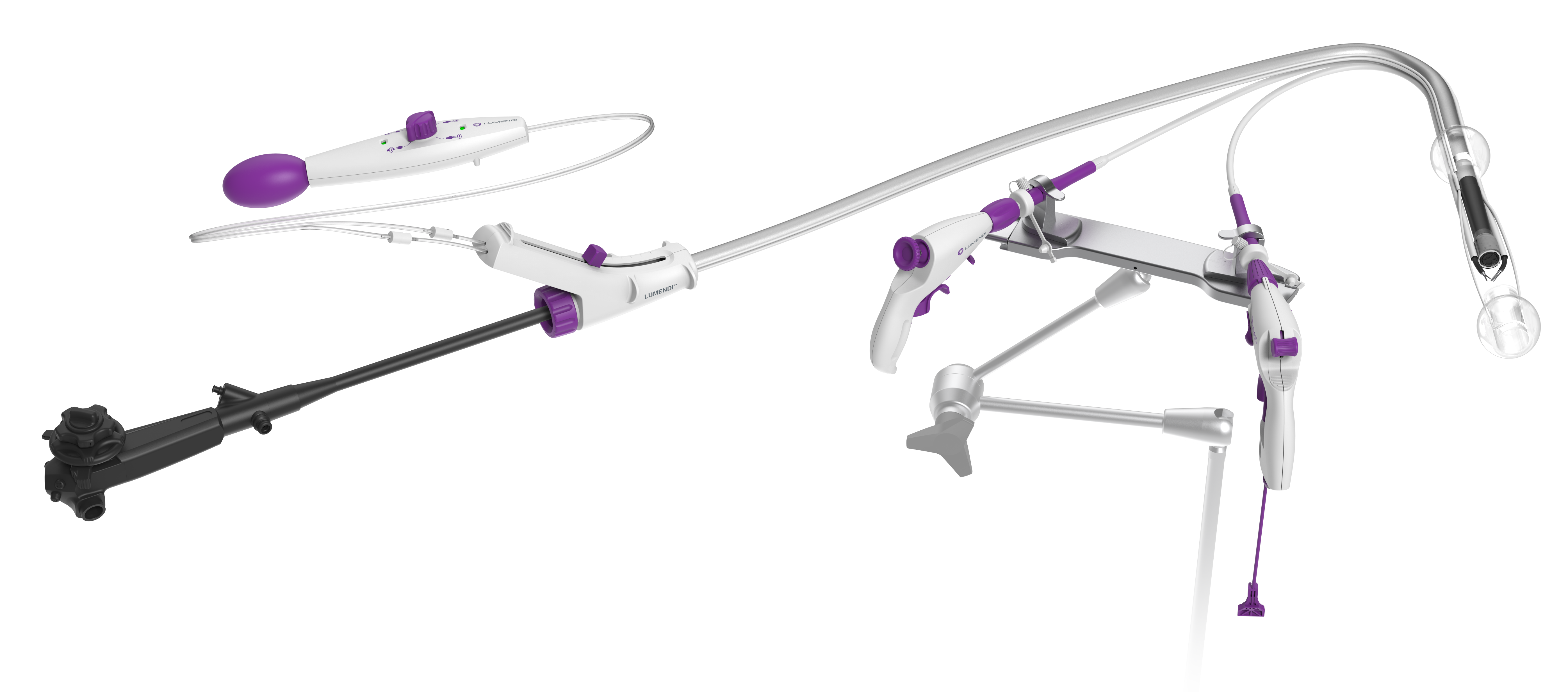
Connecticut-based medical device innovator Lumendi has announced it has received FDA 510(k) clearance for the DiLumen Is Endolumenal Interventional Scissors, a sterile, single-use, disposable, monopolar electrosurgical device for cutting, dissecting, and cauterizing tissue within the digestive tract during endoscopic procedures. This is the fourth device Lumendi has developed as the company continues to advance minimally invasive endolumenal therapies.
Lumendi’s DiLumen Is and recently 510(k)-cleared DiLumen C2 will round out a complete platform of accessories intended to improve access and therapy in the colon and reduce surgical interventions. The DiLumen C2 is a second-generation Endolumenal Interventional Platform (EIP) designed to provide complete positioning of an endoscope in the large intestine and assist with optical visualization, diagnosis and endoscopic treatment.
(Image credit: Lumendi)
Other available DiLumen accessories are the:
- Ig Endolumenal Interventional Grasper, a flexible endoscopic tool intended to grasp and manipulate tissue within the digestive tract under direct endoscopic visualization
- Im Endolumenal Intervention Mount, an ergonomically designed workstation that will support the various accessories and allow the clinician to perform procedures in a more comfortable standing or seated position
“The DiLumen platform of devices, now complemented by DiLumen Is, is designed to further facilitate and improve technically difficult and time-consuming endoscopic interventions for colonic lesions, such as polyps, a common condition that affects millions worldwide, and may replace invasive open surgical or laparoscopic procedures for patient benefit and potentially reducing healthcare costs,” says Dr. Peter Johann, CEO of Lumendi.
Adequate tissue manipulation and traction, combined with effective cutting, dissecting and cauterizing, continues to be a major challenge for therapeutic procedures in the digestive tract. These new Endolumenal Interventional Instruments are based on Lumendi’s initiative to improve access and tissue manipulation in the colon to help move many gastrointestinal surgeries to less invasive endolumenal procedures.
The DiLumen EIP consists of a single-use, soft flexible sheath that fits over standard and small-diameter endoscopes. The device employs two balloons, one behind the bending section of the endoscope and the second that can extend in front of the tip of the endoscope once at the lesion site. When both balloons are deployed and inflated, a stable Therapeutic Zone (TZ) is created. This TZ facilitates more localized insufflation and manipulation of the colon and may provide better access to lesions. With the addition of two working channels for DiLumen Ig and DiLumen Is, tissue can be manipulated, cut and dissected in a way that may improve removal of lesions during endolumenal interventions.
Lumendi also reports that, to date, clinicians have completed over 400 procedures with the commercially available DiLumen EIP with no serious device related adverse events. Three clinical studies using the DiLumen EIP have also been completed, further demonstrating safety and cost effectiveness. The studies are expected to be published in the near future.




