Connecticut-based medical device innovator Lumendi, LLC http://www.lumendi.com has announced it has received United States Food and Drug Administration (FDA) 510(k) clearance for the DiLumen™, an endoscopic accessory that is indicated to ensure complete positioning of an endoscope in the large intestine and assist with optical visualization, diagnosis and endoscopic treatment.
DiLumen is a pioneering technology that stabilizes a colonoscope to better facilitate incision-free endolumenal therapeutic procedures, which are complex procedures utilizing a flexible colonoscope and therapeutic devices inserted into the colon to locate and treat diseased tissue while preserving the colon. The device was developed by Lumendi in collaboration with the Minimally Invasive New Technologies program (MINT) at Weill Cornell Medicine and New York-Presbyterian http://www.mint.weill.cornell.edu It is the first device from the Endolumenal Interventional Platform based on Lumendi and MINT’s initiative to migrate many gastrointestinal surgeries to endolumenal procedures.
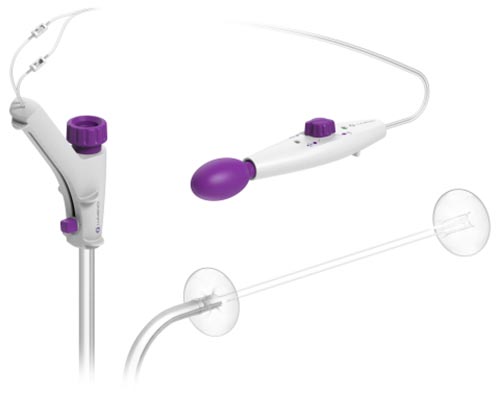
DiLumen™ (Credit: Business Wire)
According to Dr. Peter Johann, CEO of Lumendi, Ltd., “DiLumen is the first step in a family of devices to enhance endoscopic treatment, including many promising endolumenal therapeutic procedures, that may ultimately improve patient care. Lumendi sees a great potential in endolumenal interventions and is committed to build on this opportunity.”
DiLumen facilitates endoscopic treatment of colonic lesions such as polyps, a common condition that affects millions worldwide. Such treatments may take the place of open surgical or laparoscopic procedures, potentially reducing healthcare costs.
DiLumen consists of a single-use, soft flexible sheath that fits over standard and small-diameter colonoscopes. The device employs two balloons, one behind the bending section of the colonoscope and the second in front of the tip of the colonoscope. When both balloons are deployed, and inflated, a stable Therapeutic Zone (TZ) is created. This TZ facilitates more localized insufflation and manipulation of the colon and provides improved access to lesions to enable endoscopists and surgeons to perform precise endolumenal interventions. Once the procedure is complete, the balloons are deflated and removed along with the colonoscope.
“DiLumen’s use during flexible colonoscopy is an important technical advance in a field that has previously been defined by laparoscopic and open surgical procedures. DiLumen can stabilize a section of colon and facilitate the endoscopic removal of complex adenomas or polyps, with the potential to positively impact patient outcomes. Although reporting of results in patients awaits clinical studies, we are extremely optimistic that this technology will be transformative in treating digestive diseases,” said Jeffrey Milsom, M.D., Chief of Colorectal Surgery at New York-Presbyterian/Weill Cornell Medical Center and Co-Director of the MINT Program, and the Jerome J. Decosse, MD, Distinguished Professor of Surgery at Weill Cornell Medicine. Dr. Milsom is a co-inventor of DiLumen and a paid member of a clinical advisory board for Lumendi, Ltd.




