Luminopia announced today that it received FDA de novo approval for its prescription therapy for improving vision in children with lazy eye.
Luminopia announced today that it received FDA de novo approval for its prescription therapy for improving vision in children with lazy eye.
Cambridge, Massachusetts-based Luminopia’s prescription Luminopia One holds indication for improvement in visual acuity in children between ages four and seven with lazy eye (amblyopia) associated with anisometropia and/or mild strabismus, having received treatment instructions as prescribed by a trained eye-care professional, according to a news release.
“The FDA approval of a new digital therapy with robust clinical evidence for children affected by amblyopia is a major development. Amblyopia is one of the most common conditions I manage as a clinician, and patients, parents and physicians often struggle with current therapies,” Boston Children’s Hospital Ophthalmologist-in-Chief & Richard Robb Chair in Opthalmology & advisor to Luminopia Dr. David G. Hunte said in the release. “The idea of prescribing TV shows and movies to treat amblyopia in children instead of eye patches or eye drops is an exciting prospect.”
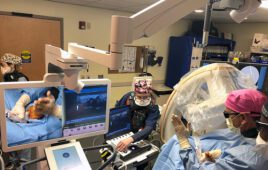
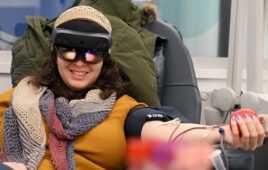
The Luminopia One platform allows patients to watch therapeutically modified TV shows and movies to improve vision within a virtual reality (VR) headset. The company said it is the first FDA-approved digital therapeutic for children with amblyopia and, more broadly, the first for a neuro-visual disorder.
Luminopia One offers more than 700 hours of popular, engaging and educational content from which users can choose, with proprietary algorithms included to modify the selected videos in real-time within a VR headset to promote weaker eye usage and encourage patients’ brains to combine input from both eyes.
“We’re proud to be part of the FDA’s groundbreaking decision today, to approve a first-of-its-kind digital therapeutic that allows patients to watch their favorite TV shows and movies to improve their vision,” Luminopia CEO Scott Xiao said. “We would like to thank our partners, advisors, study participants and team members for getting us here, and we are excited to bring Luminopia One to children with amblyopia across the country. This important milestone also opens the door for us to adapt our technology to create engaging digital therapeutics for other neuro-visual disorders.”
Luminopia expects to launch the platform in the second quarter of 2022. The prescription of the therapeutic will be for at-home use at a clip of one hour per day, six days per week, over 12-week periods.
The company received approval thanks to positive data from multiple clinical trials, including its recently published Phase 3 pivotal trial that demonstrated safety and efficacy in 105 randomized children. At the 12-week primary endpoint, the weak eye visual acuity of patients in the treatment group improved 1.8 lines on average on a logMAR eye chart, compared to a 0.8-line improvement in the control group, proving statistically significant.