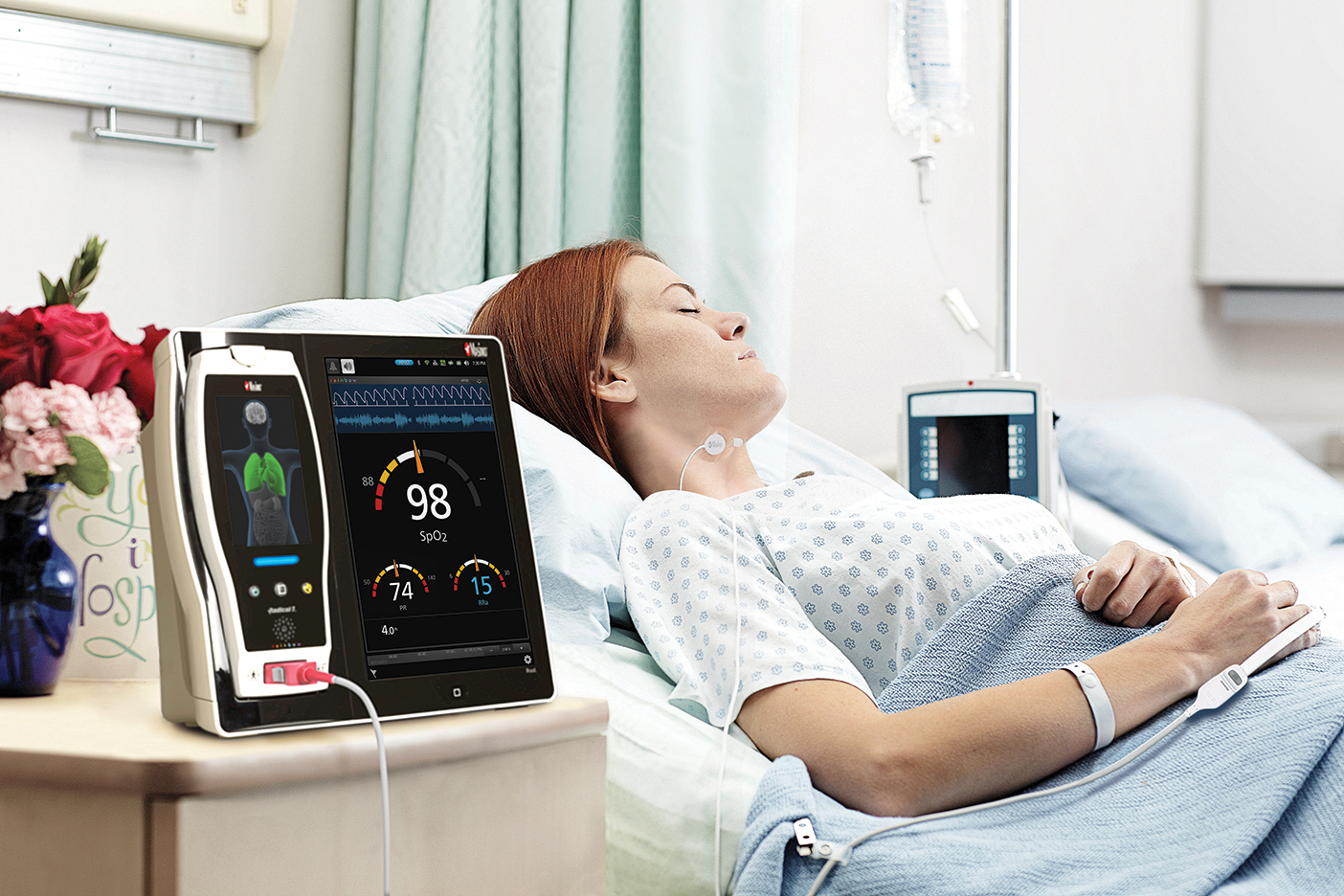
Masimo recently announced the CE Marking of RAS-45, a single-use adult and pediatric acoustic respiration sensor for rainbow Acoustic Monitoring (RAM) of respiration rate (RRa).
Continuous monitoring of respiration rate is especially important for post-surgical patients receiving patient-controlled analgesia for pain management. The Anesthesia Patient Safety Foundation (APSF) and The Joint Commission recommend continuous oxygenation and ventilation monitoring for all patients receiving opioid-based pain medications. RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer, such as Masimo’s RAS-125c and now RAS-45, that is applied to the patient’s neck. Using acoustic signal processing that leverages Masimo’s breakthrough Signal Extraction Technology (SET), the respiratory signal is separated and processed to display continuous acoustic respiration rate (RRa). RRa has been shown to be accurate, easy-to-use, and reliable, and to enhance patient compliance. RRa may facilitate earlier detection of respiratory compromise and patient distress, offering a breakthrough in patient safety for post-surgical patients and for procedures requiring conscious sedation.
(Image credit: Masimo)
RAS-45 is designed to facilitate placement on and improve attachment to the neck, but with a smaller adhesive profile than the RAS-125c. It is flexible and uses a transparent adhesive. Like the RAS-125c, it operates with Masimo MX technology boards to measure RRa and display the acoustic respiration wave form. RAS-45 maintains the same performance parameters, range, and accuracy specifications as RAS-125c. Both sensors are for patients who weigh more than 10 kg.
Joe Kiani, Founder and CEO of Masimo, comments, “RAM harnesses the power of our breakthrough signal processing technology, using Masimo SET and rainbow technologies, and applies those achievements to a respiratory measurement derived from the sound of breathing. Studies have found that RAM RRa is more sensitive to detecting respiratory pause and yet easier for clinicians and patients to use.”




