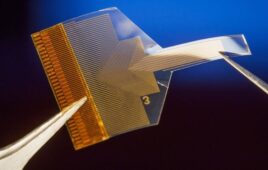
Masimo has announced that its RD SedLine EEG sensor is now available in the U.S. The EEG sensor is meant to be used with Masimo SedLine Brain Function Monitoring and is compatible with the O3 Regional Oximetry offered by Masimo.
Masimo has announced that its RD SedLine EEG sensor is now available in the U.S. The EEG sensor is meant to be used with Masimo SedLine Brain Function Monitoring and is compatible with the O3 Regional Oximetry offered by Masimo.
The SedLine and O3 monitor onto the Masimo Root monitoring platform to help clinicians obtain more information about the brain.
RD SedLine EEG sensor has a repositioned, color-coded sensor-cable connection that is positioned comfortably on the patient’s head. The soft foam pads reduce discomfort when patients are using the sensor. The performance and specifications of the sensors are the same and use existing SedLine modules through an updated patient cable.
The brain monitoring functions offered by the SedLine EEG sensor feature four simultaneous EEG leads that enable continuous assessment of both sides of the brain. It also has 4 EEG waveforms, a Density Spectral Array and the Patient State Index. The Patient State Index has been enhanced in this version to make it less susceptible to electromyographic interference and to improve low-power EEG performance.
Near-infrared spectroscopy continuously monitors absolute and trended regional tissue oxygen saturation in the cerebral region in the O3 regional oximetry.
Regional oximetry can help clinicians monitor the amount of oxygen in the cerebral region in situations that pulse oximetry may not work.
“Root with SedLine and O3 presents a powerful brain monitoring solution,” said Joe Kiani, founder and CEO of Masimo, in a press release. “With the addition of the RD SedLine EEG sensor, the EEG and optical sensors fit together like puzzle pieces, making it easier for clinicians to simultaneously monitor patients with both technologies, while providing a comfortable experience for the patient.”
The next-generation SedLine has yet to receive FDA 510(k) clearance and is not available in the U.S.
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]