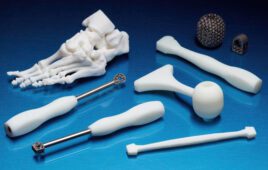
Materialise said today it won FDA 510(k) clearance for its Mimis inPrint software designed for 3D printing anatomical models for diagnostic use, touting it as the first clearance of its kind.
The Belgian company said that last August, the FDA ruled that software designed to create files for 3D printing patient-specific anatomical models for diagnostic purposes would be labeled as class II medical devices, and that it is the first company to provide conforming software to create such models.