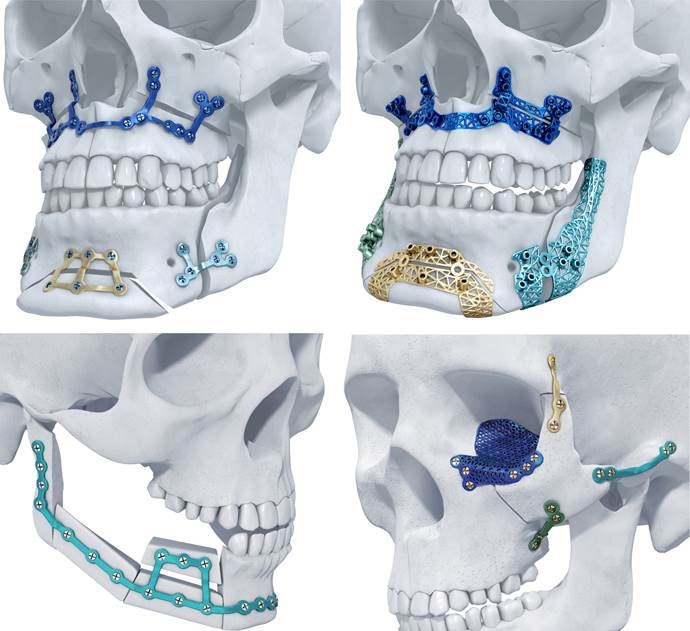
Materialise recently announced that U.S. patients can now benefit from their patient-specific implants, 3D-printed in titanium for maxillofacial surgery. As the first 3D-printed titanium maxillofacial implants to receive clearance for the U.S. market, the decision marks a further extension to Materialise’s longstanding collaboration with DePuy Synthes, who will distribute the device in the U.S.
The TRUMATCH titanium 3D-printed implants are part of a personalized total solution for orthognathic surgery, also known as corrective jaw surgery, as well as for facial reconstruction. From virtual surgical planning to the 3D-printed implants used in combination with 3D-printed surgical guides, the system can help achieve better aesthetic results and minimize surgery time for patients. It has achieved positive results in European and Australian markets since its introduction in 2016.
These are the first 3D-printed titanium maxillofacial implants to receive clearance for the U.S. market, making 3D printing technology more accessible than ever for the U.S. healthcare industry. The solution relies on the Materialise backbone of services, tapping into their software development, clinical engineering, and 3D printing production facilities.
(Image credit: Materialise)
“As the first of our extensive selection of implants to receive clearance for the U.S. markets, the decision is a real milestone for our medical department,” says Brigitte de Vet, vice president of Materialise Medical, “Thanks to our partnership with DePuy Synthes, our devices will be able to provide better healthcare for as many patients as possible.”
“For seven years now I’ve experienced the benefits of 3D-printed implants firsthand — they simplify maxillofacial surgery and allow me to perform procedures more accurately, saving time in the OR and improving patient outcomes,” says Dr. Thomas Schouman, CMF surgeon at Groupe Hospitalier Pitié Salpêtrière, France. “Moreover, they offer new treatment possibilities, allowing me to perform more complex surgeries or multiple procedures in a single intervention whereas without the implants several interventions would be necessary.”




