By Paul Dvorak, Founding Editor

The four devices, made of Evonik’s Resomer polymers, are intended to replace some stainless steel screws used to hold bone fragments together while they heal. As the screws are absorbed by the body over a period of months, bone regenerates to fill the holes once occupied by the screws.
Image courtesy of Evonik Coporation
Imagine a material with the strength of steel but the density of plastic, or synthetic skin printed on demand in the ER for a badly burned patient. Materials like these may soon be ready for prime time – and more are on the way.
Titanium and its alloys were once considered exotic materials in the medical device world. Today they’re old hat – peek in a few labs and R&D facilities and you’ll the truly exotic, materials used in electronic fingers capable of detecting tumors as deep as 20 mm and as small as 5-mm diameter, and fracture repair putty that stimulates bone growth. Researchers are even developing a biomaterial that can be mixed with a drug, then injection-molded into anatomically useful shapes.
If that’s not remarkable enough, consider the cutting-edge research lab run by the U.S. Army, the Defense Advanced Research Projects Agency, or DARPA ,and its long wish list for medical innovations for the battlefield. These are just a few of the ideas finding their way into materials and manufacturing cleanrooms today. Here are some we found a few that deserve close watching.
Bioabsorbable
This is the big news. Materials that are bioabsorbable – they’re absorbed and excreted by the body over time – would be noteworthy enough. But now let it carry a drug as well, and the combination becomes more potent.
“This is the most exciting material development we have ever encountered,” says MTD Micromolding President Dennis Tully. His company provides the manufacturing expertise for small medical components. “The wide property range of the bioabsorbable materials and their ability to carry drugs opens a door to many possibilities. And the material properties can be adjusted, from stiff to flexible. These allow conquering problems that nobody has a solution to, until now.”
One such material, Resomer, a family of lactide and glycolide based bioresorbable polyester polymers, comes from material development company Evonik Industries AG. Using advanced techniques, these polymers are further formulated into medical devices and extended-release drug delivery systems.

Can you identify this? It’s the business end of a needle, loaded with Evonik’s drug-loaded particles. Ordinarily, the particles would be carried in a fluid for injections.Image courtesy of Evonik Coporation
Doctors and patients prefer an implant made from the fully resorbable polymer over traditional nondegradable biomaterials such as metals, ceramics, and composites when the therapeutic properties of the implant are only needed for a period ranging from weeks to months. Another plus: A resorbable polymer lowers costs and the risk of infection because there is no need to remove the implant in a follow-up procedure. Evonik says medical implants made from its bioresorbable polymers degrade completely and are excreted from the body, leaving no trace behind. Typical applications include sports medicine, trauma, CMF, coronary stents, drug delivery, regenerative medicine and medical coatings.
A remarkable feature of these materials is that the degradation period is controllable depending upon application. “Degradation is controlled with the monomer ratio and other factors, such as microstructure of the polymers which controls the release of the drug, inherent viscosity ratios, molecular weight, or end groups of the polymers,” says Paul Spencer, head of Evonik’s Global Biomaterial Business. “In addition, it is possible to functionalize the polymers to do different things. So a lot of technology surrounds knowing how to tailor the polymers for specific medical device applications or to meet the needs of the drug and the release requirements from the client.”
According to medical device developer Abbott Vascular, one application for the material is a bioabsorbable polymer scaffolding – commonly known as a stent. Abbott Vascular says its Absorb bioresorbable vascular scaffold is the first drug-eluting, fully biodegradable scaffold on the market for treating coronary artery disease. The device is available in Europe, but not yet in the U.S.
“The materials are also used as coatings on cardiovascular stents. A lot of products such as metal stents have a drug-eluting coating that is completely biodegradable. The coatings can be derived from Resomer polymer products,” adds Spencer.
Developing a new material is just the first step. Next one needs a method for turning the material into useful shapes.
“Along with injection molding and extrusion, Evonik has the know-how to process and formulate the material into microparticles and nanoparticles with drugs to provide extended delivery, over weeks or months depending on the drug and the indication,” adds Spencer.
Microparticles average about 80 microns, but with nanoparticles shrink that figure to around 100 nm or smaller. The diameter can be controlled for the drug and application.
When microparticles are injected into muscle, the muscle encapsulates the spheres, which draw in moisture and swell to keep themselves in place, Spencer explains.
“And because the particles degrade by hydrolysis, the drug is released as they absorb water. What’s more, utilizing Evonik’s FormEZE manufacturing process, they can be delivered by a narrow-gauge needle – and that is significant because it’s less painful than larger needles, especially for patients who might receive injections on a regular basis,” he says.
Spencer says Evonik’s Resomer business has invested in a state-of-the-art facility to manufacturer these drugs and polymers for clients. These could be standard and high-potent drugs, cancer drugs, and the technology is really not therapeutically limited, he says.
FormEZE is the company’s proprietary process for combining the polymers with drug and make them into a microparticles or nanoparticles. Historically, the particle technology came out of MIT in the late 1980s.
In a message to device developers, MTD Micromolding’s Tully suggests that smaller, specialized material companies can dial in properties that an application needs and provide test materials.
Larger firms are ready to help too, says Evonik’s Spencer. In 2014, the company opened what it calls the MedTech Project House for developing new products suited for medical technology in Birmingham, Ala. Evonik opened the facility with 25 scientists and engineers and released them from day-to-day business with the purpose of designing and engineering the next generation of materials and processes for the medical device industry.
“If a device company wants a high-strength Resomer for an orthopedic application, the Project House researchers would work on that, and develop the process to fabricate the material. So we can introduce a new material and provide processing directions,” says Spencer, noting that the research will focus on a range of products, such as adhesives, composites, stronger biodegradable materials, or materials with different features and properties.
A fix for severed spinal cords?
Online reports of treatments for spinal cord injuries are not encouraging. It seems generally accepted that central-nervous-system breaks cannot be rejoined. That thinking makes the work of the company TissueGen all the more significant. The company reports that a U of Texas research team using a company development is working to overcome the limitations associated with incorporating 3D chemotactic gradients in nerve-repair scaffolds. Nerve guides are created using the company’s Elute BDL fibers that are loaded with neurotrophic agents (having an affinity for the nervous system) wound in a variable-pitched coil for what the company says are highly tunable, 3D neurotrophic gradients. The team has demonstrated that these gradient nerve guides created longer axons (the usually long process of a nerve fiber that generally conducts impulses away from the body of the nerve cells) and more direct (linear) path of axons compared to similar nerve guides with linear neurotrophic concentration in an ex vivo dorsal-root-ganglion model.
TissueGen is applying the technology to develop a medical device targeting treatment and recovery of patients with spinal-cord injuries. Gradient scaffolds may eventually be surgically implanted into a severed spinal cord to entice motor and sensory axons to regrow, and may aid in the recovery of what were previously considered irreparable injuries.

This sample of synthetic skin was printed by a device developed by students and instructors at the University of Toronto.
Printed synthetic skin
Printing replacement human organs, a research goal for decades, has become a reality in recent years. About nine years ago, the transparent image of a printed human heart adorned the cover of one medical magazine. The image suggested the possibilities of a functioning organ made of tissue, not hard plastic.
A few years ago, under the leadership of associate professor Axel Geunther, researchers at the University of Toronto devised a way to print an epidermal layer over a dermal layer, just like natural skin.
Apparently DARPA threw down a gauntlet about that time, seeking a ready replacement to help burned and wounded soldiers recover more quickly and with less scarring. The team tackled that problem by finding a way to modify conventional print reservoirs so they would jet skin cells. Cartridges were devised to carry either epidermal or dermal cells. Working with burn surgeon Dr. Marc Jeschke of Toronto’s Sunnybrook Research Institute, the team produced the specialized cartridges and fluid to keep the skin cells alive. During printing, the cells gel into a biodegradable dressing.
So far, the team has printed portions of human skin that helped the wounds on immune-compromised mice heal. The next step would include experiments with pigs and larger sizes of tissue.
Electronic fingers
Tumors can be difficult to detect by touch, but that’s a common initial discovery method. This is especially true of breast tumors, but there even reading a clear X-ray takes some experience.
A team of researchers at the University of Nebraska, led by Professors Ravi Saraf and Chieu Van Nguyen, have devised a sensitive film that can sense differences in tissue density in human breast tissue, which can be an indicator of tumors.
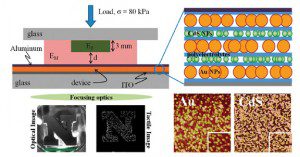
From their paper on the film’s capability, Saraf and Nguyen say their tactile device detects a 5- to 10-fold stiffer object imbedded as deeply as 20mm deep in a softer material. The light emitted from the film is linearly proportional to local stress. The thin-film tactile device is a multilayer composite made of nanoparticles and polymers. The film is an analog electro-optical device that shows embedded stiffness as continuous variation in light emission that can be focused on a camera. So the pictures captured represent accurate shape of the denser volumes – the tumors. And because it’s an imaging technology, it allows for the creation of digital records of examinations, something notes and recollections do not provide. Saraf says the electro-mechanical characteristics of the flexible thin film are precisely tuned to obtain a tactile image of the denser “lump” for contact pressures from 4 to 12 psi, about that of human touch.
Fracture putty
Some nuts are tougher to crack than others. One idea that easily captures attention came out of DARPA’s idea lab: Fracture putty. Ugly battlefield wounds such as compound bone fracture are difficult to treat, often requiring multiple surgeries and long healing and rehabilitation times. Current standard procedures that use screws, plates, and rods can lead to further complications.
A few researchers have taken a crack at the problem. A multi-facility team lead by Matthew B. Murphy at the Methodist Hospital Research Institute in Houston reports on a good start. Their idea is to provide a mix that includes a bioactive sponge with a composite of platelet-rich plasma, nanoporous silicon-enclosed microparticles, and adult-bone stem cells. The team devised a gel carrier to deliver and retain the stem cells and microparticles in the porous scaffold that would promote cell growth. The material was implanted subcutaneously in rats to evaluate the formation of bone tissue. Good first step: They found one combination of the ingredients formed more bone and was more vascularized after four weeks than another combination.
How to teach an “old” material new tricks
Titanium has been around a bit longer than recent polymers because inventive companies keep reinventing the material. Titan Spine, for one, has been developing titanium alloys for use in spinal interbody fusions since 2006. “We have developed the expertise to manipulate the surface to promote bone growth and fusion,” said the company’s VP of Marketing Andrew Shepherd. Surface technology, he adds, is the science of surface manipulation to create a desired biochemical response. “In our case, we select for osteogenesis,” he says. “Titanium is the only material that has a natural affinity with bone and can be roughened with the specific textures at the macro, micro, and nano scales that favor bone growth and fusion.

Titan Spine’s Endoskeleton devices are for use in skeletally mature patients with Degenerative Disc Disease and with or without grade 1 spondylolisthesis or retrolisthesis. The roughened surface, a proprietary feature developed by the company, encourages bone growth.
Over the past decade, the material polyetheretherketone (PEEK) has been marketed as a better material for interbody fusion devices because it has a lower modulus of elasticity than titanium and is closer to the modulus of bone. “Some asserted that PEEK devices would have lower rates of subsidence – collapsing in to the bone – than titanium devices,” he says. However, any biomechanical engineer knows the key variable in avoiding subsidence is the structural stiffness of the device rather than the implant’s material modulus. A recent study showed that one of Titan’s devices subsided at statistically reduced rates and overall amounts as compared to a commercially available PEEK device. He suggests that design engineers should let science guide the development of innovative and beneficial medical devices rather than fallacious marketing.
All Titan Spine devices are made from titanium alloys and feature a proprietary combination of roughened textures at the macro, micro, and nano scales created through a subtractive process. “It’s not a coating,” he says. “The macro roughness is designed to resist expulsion, while the micro and nano roughened features interact with stem cells to upregulate osteogenic (bone forming) and angiogenic (blood vessel forming) growth factors necessary for bone growth and, ultimately, fusion.
He adds that the company will be launching a next-generation surface, called nanoLOCK, later this year that features even more nano-scaled textures that improve the osteogenic response to the company’s interbody fusion devices. “nanoLOCK is the first nanotechnology cleared by the FDA for spinal applications,” he says.
What plastic is implantable? Most of them, with a caveat
One of the more surprising things, says MTD Mirco Molding’s Dennis Tully, is that when discussing new projects with clients and engineering groups, even those in large companies with huge R&D budgets, it usually comes as a surprise that almost any plastic material can be used in an implant.
“Most are shocked to hear this. However, qualifying a material for in vitro use calls for proper testing. And there is a lot of misinformation about whether or not a material must be tested for a new application, especially when the material has proven itself in another application. They ask, ‘Can we forego the testing?’ And the answer is most always, ‘No,’” says Tully.
“But most people don’t understand that trying to leverage someone else’s data does not work most times. That is because it was not generated in the same process. So there is a wide interpretation of the rules specified by the FDA regarding material.
“There is also a great misunderstanding about what predicated data can be used relative to materials and whether or not a new material can be qualified. When the material has the right fit for the application, most often you find you have to start from square one no matter what material you select,” explains Tully.
The hazard is that product developers discover the need for retesting when they are far into development and don’t want to consider starting over. His take-away thought:
Most materials can be used, but they must be tested to FDA requirements.