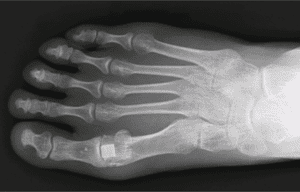
The Cartiva SCI is the first hydrogel polymer implant to replace a joint articulating surface ever approved by the FDA and the first PMA for any product in the forefoot.
Musculoskeletal Clinical Regulatory Advisers (MCRA) announced its role in the successful Premarket Approval (PMA) application decision by the U.S. FDA to approve Cartiva’s Synthetic Cartilage Implant (SCI) for the treatment of osteoarthritis in the first metatarsophalangeal (MTP) joint (the “great” or “big” toe).
The Cartiva SCI is the first hydrogel polymer implant to replace a joint articulating surface ever approved by the FDA and the first PMA for any product in the forefoot. This is the ninth successful PMA in which MCRA has assisted a client since 2006, including the only three lower extremity PMAs.
MCRA was retained in August 2015, following the original PMA submission in May 2015, to work in conjunction with Cartiva on FDA interactions, responses to FDA questions and preparation for a meeting of the Orthopedic and Rehabilitation Devices Advisory Panel. Following a positive panel vote on the safety and effectiveness of the device on April 20, 2016, the FDA approved the PMA for the Cartiva SCI on July 1, 2016.
Musculoskeletal Clinical Regulatory Advisers
mcra.com
Cartiva
cartiva.net




