
Solvent-based vapor degreasing removes production soils. [Photo courtesy of MicroCare]
What comes first: the vapor degreaser or the cleaning fluid? That depends on the contamination and the best cleaning process for it.
Elizabeth Norwood, MicroCare
Cleaning is an essential process in the production of medical devices and equipment. It helps ensure parts meet quality and validation standards, but also helps guarantee device reliability.
When it comes time for medical parts manufacturers to upgrade their cleaning methods, it’s important to do some legwork before purchasing any cleaning fluids or vapor degreaser cleaning equipment. Without proper planning, you might find yourself with a new — and expensive — piece of cleaning equipment but no compatible cleaning fluid or method to get the job done.
To avoid this dilemma, three questions need to be answered first.
1. What is the contamination?
Knowing the type of contamination helps narrow down your cleaning fluid selection.
Medical device parts contamination encompasses a number of production soils, including hydrocarbon-based stamping oils, machining lubricants, corrosion protection agents and metal shavings.
They also include more stubborn soils like solder fluxes, silicone oils, synthetic greases, grinding media, polishing pastes, baked-on resins, inks, heavy pitches and thick waxes.
2. What cleaning process should be used?
When vapor degreaser cleaning, the parts contamination also helps determine which cleaning method to use.
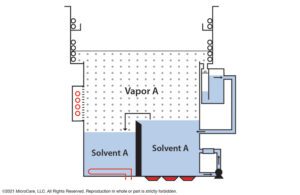
Mono-solvent vapor degreasing uses a single cleaning fluid inside a traditional two-sump vapor degreaser. [Image courtesy of MicroCare]
Some mono-solvents are also azeotropes, a cleaning fluid made with two or more components that combine to form a stable mixture. They do not separate and always behave as one solvent, even during boiling, cooling and distillation. Azeotropes help increase a fluid’s cleaning performance, make it nonflammable or improve its toxicity profile. They boast the cleaning performance and safety of a mixture of cleaning solvents but are easier to store, handle and dispose of.
Co-solvent vapor degreasing delivers the speed and convenience of mono-solvent cleaning but uses two solvents for more cleaning power. A very high temperature boiling fluid in one sump and much lower boiling temperature fluid in another work together to boost cleaning performance. The vapor degreaser can also be outfitted with sprayers, super-heat, ultrasonics and other add-on accessories to enhance cleaning performance. Co-solvent vapor degreasing is ideal for removing more stubborn soils.
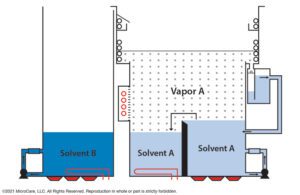
Bi-solvent vapor degreasing uses two solvents for more cleaning power. [Image courtesy of MicroCare]
Warning: Proceed with caution when opting for a co-solvent or bi-solvent vapor degreasing cleaning method. Not all medical device parts require these more aggressive, high-temperature cleaning methods. Using a very powerful co-solvent or bi-solvent could damage delicate substrates, especially the acrylic, ABS and polycarbonate commonly used in many medical devices and parts.
3. What is the best cleaning fluid to use?
Environmental regulations should also guide cleaning engineers on which vapor degreasing fluid is the right one use. It must meet local, state or federal regulatory requirements.
For example, commonly used nPB (n-Propyl Bromide) and TCE (trichloroethylene) are either banned or under scrutiny in many countries due to air and water quality concerns. This means that these cleaning fluids will likely need to be replaced with better alternatives soon and should probably be avoided.
Fortunately, a number of effective cleaning fluids on the market today boast zero ODP (ozone-depleting potential) and low GWP (global warming potential) ratings to meet increasingly stringent environmental regulations.
Stay bioburden-free
No matter which vapor degreasing fluid or method a cleaning engineer chooses they can rest assured that the modern solvent-based fluids are hostile to bioburden and don’t harbor pyrogens. Vapor degreasing with solvents does not use water, which helps maintain an environment free of bacteria, viruses, or other pathogens. It is also a convenient way to validate a cleanroom-compatible, bioburden-free cleaning process.
Choose the fluid, then the vapor degreaser
It is important for the cleaning engineer to first understand the type of contamination and which vapor degreasing method and fluid will best remove it. Then, armed with this information, they can choose the appropriate vapor degreasing equipment and accessories.
For example, if a cleaning engineer purchases a vapor degreaser designed for mono-solvent cleaning only, the machine doesn’t allow for options. If the mono-solvent cleaning process is ineffective on their contaminant, then the engineer is left with soiled parts and an expensive vapor degreaser they can’t use.

Cleaning test labs can perform cleaning trials on dirty parts. [Photo courtesy of MicroCare]
Rely on medical device cleaning experts
It is highly recommended that cleaning engineers seek the advice of an experienced cleaning fluid supplier in order to choose the right cleaning fluid and method for their particular medical device parts cleaning challenge. Fluid suppliers often have cleaning test labs for cleaning trials on dirty parts to determine the contaminant type and recommend if the mono-solvent, bi-solvent or co-solvent cleaning process will work best. The testing also helps determine if special vapor degreaser add-ons or modifications might be necessary to get the specified level of clean.
When deciding how to clean medical devices and parts, choose the cleaning fluid first. Understanding the soils encountered, the cleaning method needed and expected cleaning outcomes up front helps medical device manufacturers know which cleaning fluid to choose and ultimately which vapor degreaser features are required.

Elizabeth Norwood is a senior chemist at MicroCare. [Photo courtesy of MicroCare]
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of MedicalDesignandOutsourcing.com or its employees.




