 Medtech companies paid $3.62 billion for access to physicians — 10% more than drug companies did — from 2014 to 2017, according to new research.
Medtech companies paid $3.62 billion for access to physicians — 10% more than drug companies did — from 2014 to 2017, according to new research.
Device company payments to surgical specialists were significantly more tied to the surgeons’ Medicare billing than payments from drug companies to specialists, according to the study, which appears in the April 2021 edition of Health Affairs.
Payments totaling more than $2.3 billion by 10 medical device firms accounted for more than two-thirds of all device payments to physicians, researchers from the University of Pennsylvania and Columbia University found. Those companies were: Medtronic, Zimmer Biomet, Johnson & Johnson, Stryker, Abbott, Arthrex, Smith & Nephew, Boston Scientific, Intuitive Surgical and Nuvasive. The researchers gathered their information from Medicare Open Payments data.
Approximately 75% of neurosurgeons and orthopedic surgeons received payments from device vendors, and those payments were higher than what non-surgical specialists were paid, the researchers found. Payments from device firms were more often related to product development and training than payments from drug companies.
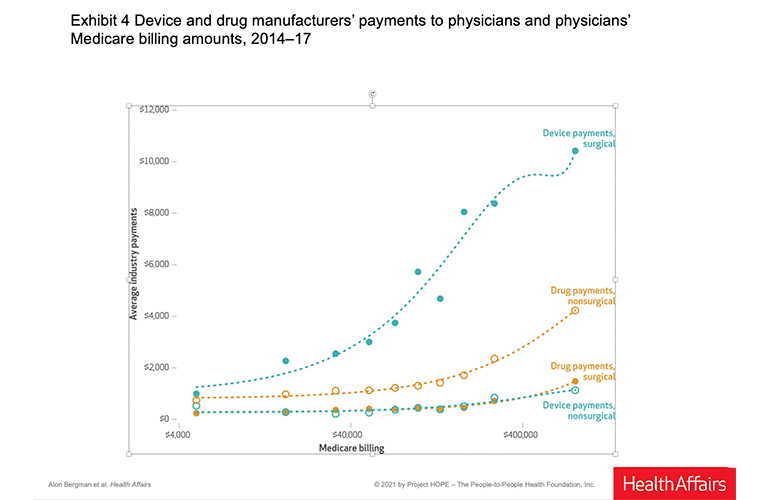
(Graphic courtesy of Health Affairs)
“While firm payments are typically of small value relative to physicians’ salaries, they can shed light on the intensity and frequency of interactions between firms and physicians,” study co-author Alon Bergman wrote in a University of Pennsylvania blog post. “Medical device firm representatives often develop close relationships with physicians. They provide education and technical assistance in operating rooms, and they seek regular feedback on products. Such interactions may facilitate training on the use of existing products and innovation of new products, but they may also represent opportunities for firm representatives to influence physicians’ treatment decisions.
“We need further research on how these close relationships between device makers and physicians relate to physicians’ choices of products, the prices paid for these products, the development of new breakthrough medical products, and device market concentration,” Bergman added. “Increased data transparency, like requiring unique medical device identifiers in medical claims, will be critical to these efforts.”
“Medical device companies rely on partnerships with doctors at every step of the innovation process,” said Scott Whitaker, CEO of the trade group AdvaMed in an email to Medical Design & Outsourcing. “Doctors help develop and refine medical devices, and they even create new devices themselves, sharing their intellectual property with companies to help save and improve patients’ lives. Then, doctors often assume primary responsibility for training other doctors on how to use these new devices safely and effectively. Unlike a pill or injection, procedures to implant or equip medical devices for patients can be extremely technical and complex.
“This kind of collaboration between medtech and providers is critical, as working directly with doctors who provide medical and scientific advice helps to ensure patients have the highest quality care borne out of real-world experience in the doctor’s office and operating room,” Whitaker added. “Still, as this study suggests, it’s important to ensure that this relationship is appropriate. That’s why more than a decade ago AdvaMed created, and then recently updated, its industry-leading Code of Ethics on Interactions with Health Care Professionals. All companies who sign the code – the majority of AdvaMed’s membership so far – commit to interacting with doctors with integrity, respect, and transparency.”
This article has been updated with comments from AdvaMed.
“AdvaMed and the medical technology industry as a whole will continue to uphold the highest ethical standards, so that our healthcare system can continue give patients the best possible health care.”

![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)


