The development of the product development plan is an evolutionary effort that continues through the life of the medical device project.
Bill Betten, Betten Systems Solutions, and Tom Waddell, Waddell Group
This is the second in a series of articles that will discuss the design of innovative products in the highly regulated medical environment. The first article focused on the process framework needed to guide the overall development. If the process serves as the foundation for the development, the “plan” serves as the blueprint for development. Applying these principles to your development process will result in a faster and cheaper path to market.As a reminder, this series will focus on the definition and execution of product development activities post-funding and includes the following:
- Idea – Without it, nothing to be developed
- Process – The structure for development
- Plan – The blueprint (The article you’re reading.)
- Requirements – The details
- Regulatory / Reimbursement – Critical to the medical device space
- Verification/validation – The right product doing the right thing
The development of the product development plan is an evolutionary effort that continues through the life of the project. Field Marshal Helmuth von Moltke famously said, “No battle plan ever survives contact with the enemy.” In much the same way, a development plan is obsolete as soon as it is committed to paper (or computer). However, a plan is essential to gathering and assigning the appropriate resources to the effort, particularly for a startup trying to obtain funding and support for the subsequent development. At Nortech Systems, we often collaborated with a client to develop a detailed plan in the proposal stage, prior to development of the requirements. This enabled the development of a more accurate proposal, as well as helped the client to understand the various elements of a complex dance among cost, schedule, and resources. This initial plan would be continuously refined throughout the project, reacting to the inevitable changes that occur along the way.
According to the Project Management Body of Knowledge (PMBOK), the plan is a formal, approved document used to guide both project execution and project control. The plan document sets planning assumptions and decisions, defines the approved scope, cost, and schedule baselines, and facilitates communication among project stakeholders. At a high level the project plan faces the traditional constraints of scope, time, and cost. Project management involves assigning resources to optimize these various constraints, often represented as a 3-legged stool with a comment that only two can be optimized at a given time. In addition, medical product development also places an emphasis on quality of both the product and the development process as well as risk (both development and product).
The plan is typically comprised of major tasks or phases associated with the overall quality system of the entity performing the work, with subtasks to varying levels of complexity. The phases correspond to the major segments of project development, ranging from development of the initial concept all the way through to project launch. An additional phase, project close-out, may be included, but should always be considered, particularly in the tightly regulated world of medical development, where product longevity and management post-launch are much more critical than in the fast-paced consumer world.
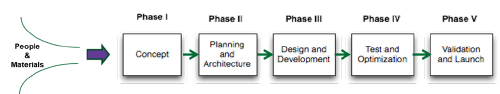
A medical device project plan includes major tasks or phases associated with the overall quality system of the entity performing the work. [Image courtesy of Betten Systems Solutions]
Within the high-level phase plan, far more detailed sub-plans exist to develop the products’ sub-components and all the associated deliverables associated with the entire product. This plan, typically done in a planning tool such as Microsoft Project, can easily consist of hundreds or thousands of interconnected items and incorporates the tasks, schedules, and resources associated with the project. While the detailed plan serves as a roadmap to success, it can also be thought of as a simulation tool, much like traditional technical tools like Finite Element Analysis, SPICE simulations, and CAD in hardware and decision tables in software. It enables assessment of “what if” scenarios as well as provides a tracking tool to show progress against the objectives. It is the key to development of the critical deliverables as well as accomplishment of the milestones along the way to the end goal.

Within the high-level phase plan, far more detailed sub-plans exist to develop the products’ sub-components and all the associated deliverables associated with the entire product. [Image courtesy of Betten Systems Solutions]
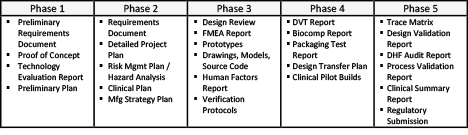
a key part of medical device development is the documentation deliverables. [Image courtesy of Betten Systems Solutions]
- Communication by the program/project manager to all stakeholders is critical. (Avoid late “surprises.”)
- Keep an eye on the big picture, manage to the critical path, and provide early warning for potentially late milestones. Don’t blindly schedule “boxes.”
- Do “sweat the right small stuff,” because it can derail you.
- Set priorities and make sure that the team knows what is “hot.” This is often based on a “critical path” analysis (a feature in most tools), that helps point out the tasks that must be completed on time to keep the schedule.
- Keep a sense of humor, a strong sense of team, and don’t panic when things happen, because they will.
Next up, let’s examine how the development of a well-defined and documented set of requirements for the product sets the stage for development of your product in a timely fashion>>
Bill Betten is the president of Betten Systems Solutions, a product development realization consulting organization. Betten utilizes his years of experience in the medical industry to advance device product developments into the medical and life sciences industries. Betten most recently served as director of business solutions for Devicix/Nortech Systems, a contract design and manufacturing firm. He also served as VP of business solutions at Logic PD, medical technology director at TechInsights, VP of engineering at Nonin Medical, and in a variety of technology and product development roles at various high-tech firms.
Tom Waddell is the CEO of Waddell Group, a consulting company dedicated to providing world class project management talent to the medical devices industry companies of all sizes. Tom was formerly a partner with Project Leadership Services, and has led more than medical device development projects.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of MedicalDesignandOutsourcing.com or its employees.