San Diego, CA – TDK-Lambda introduces the new NVM175 series of medically-approved AC-DC power supplies. Combining 4.5kVac reinforced input to output isolation with dual input fusing and an output-to-ground isolation of 1500Vac, the NVM175 meets UL/EN/IEC60601-1 and is suitable for use in BF (Body Floating) type medical applications (e.g., Ultrasound, ECG, Patient Monitors, etc.).
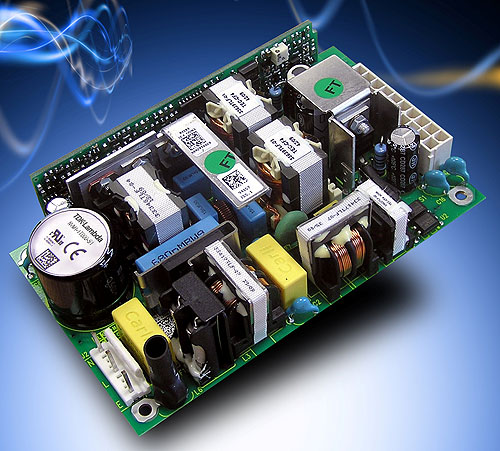
Offered in a compact 5” x 3” footprint with less than 1U height (only 1.02” high component height), the new NVM175 supplies have universal AC input and are rated at 180W at 50°C with user supplied air flow. Leakage current is a very low 80µA maximum with 120Vac/60Hz input.
With its compliance to ITE safety specifications, these power supplies are equally suita-ble for many other applications including broadcast, instrumentation, routers, servers and security networks, as well as ATE and automation equipment.
The NVM175 is available with either 12 or 24V output voltage (with 12V/0.2A standby). As well as the impressive technical performance, the NVM175 meets TDK-Lambda’s design brief for environmentally responsible products by achieving up to 90% efficiency, less than 1W standby power and RoHS and REACH compliance. Other standard fea-tures include a power good signal, remote on/off, remote sense and protection from over current, over voltage and over temperature conditions.
TDK-Lambda
www.us.tdk-lambda.com/lp
::Design World::




