(Credit: Medtronic)
Medtronic announced that the U.S. Centers for Medicare & Medicaid Services (CMS) has approved coverage for the Micra Transcatheter Pacing System (TPS).
This decision, effective immediately, follows the approval of two studies required to enable reimbursement through Medicare’s policy of Coverage with Evidence Development (CED).
In January 2017, CMS issued a final National Coverage Determination (NCD) that covered leadless pacemakers under CED when used in accordance with FDA-approved labeling in FDA-approved studies that have been approved by CMS, or under prospective, longitudinal studies approved by CMS. The two Micra studies approved by CMS are the Micra Post-Approval Study (PAS), which was required by the FDA as a condition of approval of the device in April 2016, and a new Micra CED Study, the “Longitudinal Coverage with Evidence Development Study on Micra Leadless Pacemakers,” which was developed by Medtronic to address research questions identified by CMS in the NCD.
The Micra PAS Study will enroll a subset of Medicare Micra patients through a traditional clinical research design, while the Micra CED Study will encompass all Medicare beneficiaries who receive Micra under an innovative new approach to CED, linking information from Medtronic’s device registration system to Medicare health insurance claims and enrollment data.
“Since the Medicare coverage decision was announced earlier this year, we have been working closely with CMS to secure study approvals which will provide additional evidence supporting the Micra TPS,” says John Liddicoat, M.D., senior vice president, Medtronic, and president of the Cardiac Rhythm and Heart Failure division. “We are pleased that all Medicare beneficiaries indicated for Micra according to the FDA label are now covered by the NCD and, as a result, have access to this innovative pacing technology.”
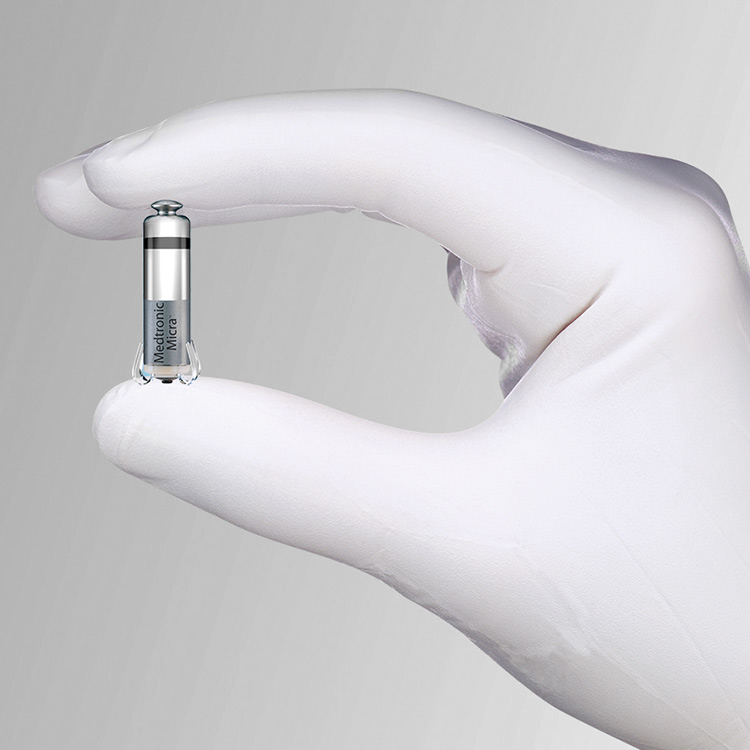
Approved by the FDA in April 2016 for patients who need a single-chamber pacemaker, the Micra TPS is the first and only leadless pacemaker approved for use in the U.S. The miniaturized device was recently named at the top of U.S. News & World Report’s list of “2016’s Biggest Achievements in Medicine.”

(Credit: Medtronic)
Comparable in size to a large vitamin, the Micra TPS is less than one-tenth the size of traditional pacemakers, yet delivers pacing technology to patients via a minimally invasive approach.
During the implant procedure, it is attached to the heart with small tines and delivers electrical impulses that pace the heart through an electrode at the end of the device.
Unlike traditional pacemakers, the Micra TPS does not require leads or a surgical “pocket” under the skin, so potential sources of complications related to such leads and pocket are eliminated – as are any visible signs of the device.
The Micra design incorporates a retrieval feature which can be enabled, if possible; however, the device is designed to be left in the body. For patients who need more than one device, the miniaturized Micra TPS can be permanently turned off, allowing it to remain in the body so a new device can be implanted without risk of electrical interaction.
The Micra TPS is approved for both 1.5 and 3 Tesla full-body magnetic resonance imaging (MRI) scans. It is designed to allow patients to be followed by their physicians and send data remotely via the Medtronic CareLink Network. Remote monitoring of Micra devices is expected to be available later this year.
Primary results from the Medtronic Micra TPS Global Clinical Trial, published November 2015 in the New England Journal of Medicine, showed the Micra TPS was successfully implanted in 99.2 percent of patients by 94 physicians around the world and that the system met its safety and effectiveness endpoints at six months follow-up with wide margins. Long-term results from the Micra Trial, published November 2016 in Heart Rhythm, reinforced these data, showing the risk of major complications at 12 months for Micra patients was low at four percent, 48 percent lower than for patients with traditional pacemakers (hazard ratio: 0.52, 95 percent CI: 0.35-0.77, P=0.001).




