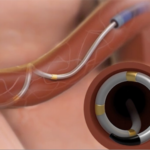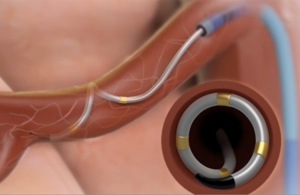
The Symplicity Spyral renal denervation system delivers energy to the nerves leading to the kidneys, which help regulate blood pressure. [Image courtesy of Medtronic]
It’s the latest development out of Medtronic’s Spyral HTN-ON MED trial testing the company’s RDN catheter system, which delivers radiofrequency energy to overactive nerves near the kidneys that cause high blood pressure. Medtronic released the results in The Lancet and at the American College of Cardiology’s 71st Annual Scientific Session.
“For the first time, we now have randomized data that demonstrates that in a typical patient population — hypertension patients who are on anti-hypertensive medications and treated with RDN — we are seeing the continued, long-term blood pressure lowering effect,” Dr. Felix Mahfoud, a cardiologist at Saarland University Hospital and a member of the SPYRAL HTN executive committee, said in a news release. “Lowering blood pressure can have meaningful clinical results for patients, including a decrease in the risk of cardiovascular events.”
The results cover the trial’s first 80 patients who were prescribed anti-hypertensive medications and randomly assigned to undergo RDN or a sham control procedure. Medtronic said the patients who had the minimally invasive RDN procedure had a clinically meaningful and lasting blood pressure reduction through three years of observation compared to the control group, while the catheter had no major device or procedural safety events through the same time.
Medtronic also said it recently closed enrollment and completed randomization of the trial’s full cohort of up to 340 patients. The six-month post-procedure follow-up for the full cohort is expected to be complete in the second half of this year, setting the stage for potential FDA approval.
“The data presented at ACC underscores Medtronic’s confidence in RDN as a solution for the millions of people who suffer from uncontrolled high blood pressure,” Jason Weidman, SVP and president of Medtronic’s Coronary and Renal Denervation business unit, said in the news release. “These new data will be important to regulatory officials, clinicians and payers as we look to bring a new treatment option to market for patients with uncontrolled hypertension.”

Medtronic’s Symplicity Spyral device is a multi-electrode catheter for renal denervation [Image courtesy of Medtronic]
Medtronic leaders have said they see a multibillion-dollar market for RDN, and some analysts remain bullish on the technology despite recent setbacks.