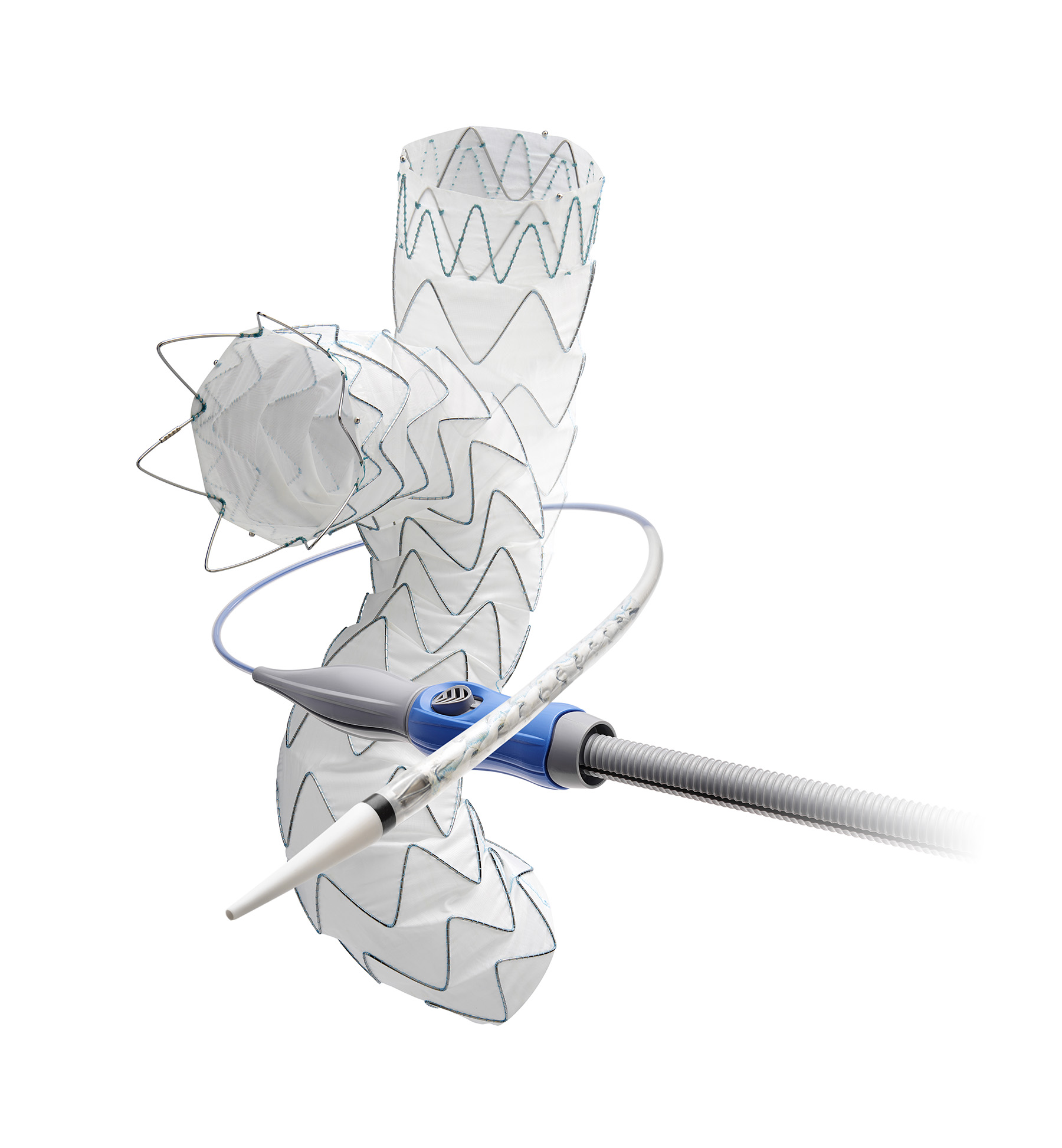
Medtronic has announced that it has received CE Mark designation for the Endurant II/IIs stent graft system to treat abdominal aortic aneurysm (AAA) patients using a ChEVAR procedure, a parallel graft chimney technique that uses commercially available balloon expandable covered stents combined with a standard aortic stent graft. This expanded indication in the European Union enables the Endurant II/IIs stent graft system to be used in patients with complex aneurysms with short aortic neck lengths ≥2 mm, expanded from the prior neck length indication ≥10 mm.
“Treating aneurysm patients with short aortic necks has been a long-time challenge for clinicians performing endovascular aneurysm repair (EVAR) to treat AAA patients,” says Professor Giovanni B. Torsello, MD, chief of vascular surgery, St. Franzkisus Hospital, Mϋnster, Germany and co-author of the PROTAGORAS study. “The availability of a standardized approach which increases anatomical applicability will help establish a new standard for patients with complex forms of AAA that may not have been suited for previous procedures.”
The CE Mark is supported by a comprehensive review of clinical data from literature using the Endurant II/IIs stent graft system with the ChEVAR technique. In the flagship PROTAGORAS study, outcomes were tracked with radiologic follow up over a mean of two years. The study used a standardized procedural approach with the Endurant system and balloon expandable covered stents. The results, which were published in the Journal of Vascular Surgery, demonstrated that standardized use of the Endurant II/IIs stent graft system with ChEVAR in 128 patients is associated with 100 percent technical success, statistically significant aneurysm sac regression (p = .001), 95.7 percent primary patency of the chimney grafts and a low incidence of chimney related reinterventions.
(Image credit: Medtronic)
“Medtronic is committed to partnering with our clinical community to provide solutions for challenging patients with complex aortic disease,” says Daveen Chopra, vice president and general manager of the aortic business, which is part of the Aortic & Peripheral Vascular division at Medtronic. “The expanded indication for our Endurant II/IIs stent graft system is a great example of how we can deliver solutions to address unmet clinical needs and improve standard of care for patients who have aneurysms with short neck lengths. We are excited to expand our leadership in EVAR with the first aortic stent graft approved for use with the ChEVAR technique.”
The Endurant II/IIs stent graft system is based on Medtronic’s leading Endurant stent graft system, which is selected for nearly one of every two endovascular AAA repairs globally and has resulted in more than 250,000 successful implants. The original Endurant system received the CE Mark in June 2008. The new expanded ChEVAR indication will be initially commercialized in Europe, and then in other countries that recognize the CE Mark approval. In the U.S., Food and Drug Administration approval for the Endurant Stent Graft System was granted in December 2010. In the U.S., the Endurant II/IIs stent system is approved for neck lengths ≥10 mm and ≤60° infra-renal angulation and it is not approved for this expanded indication.




